The carbonyl group is one of the most common functional groups. Although nucleophilic addition, reduction or reductive functionalization of carbonyl compounds are well-established, the direct “deletion” of the carbonyl group as a “CO” moiety remained very rare.1-4 What if one can directly remove a selected carbonyl from a molecular backbone? For cyclic carbonyl compounds such as cyclic ketones, lactones or lactams, this is effectively a one-carbon ring-contraction reaction. Such a reaction holds tremendous potential in molecular editing chemistry,5 given the ubiquity of carbonyl group in organic molecules.
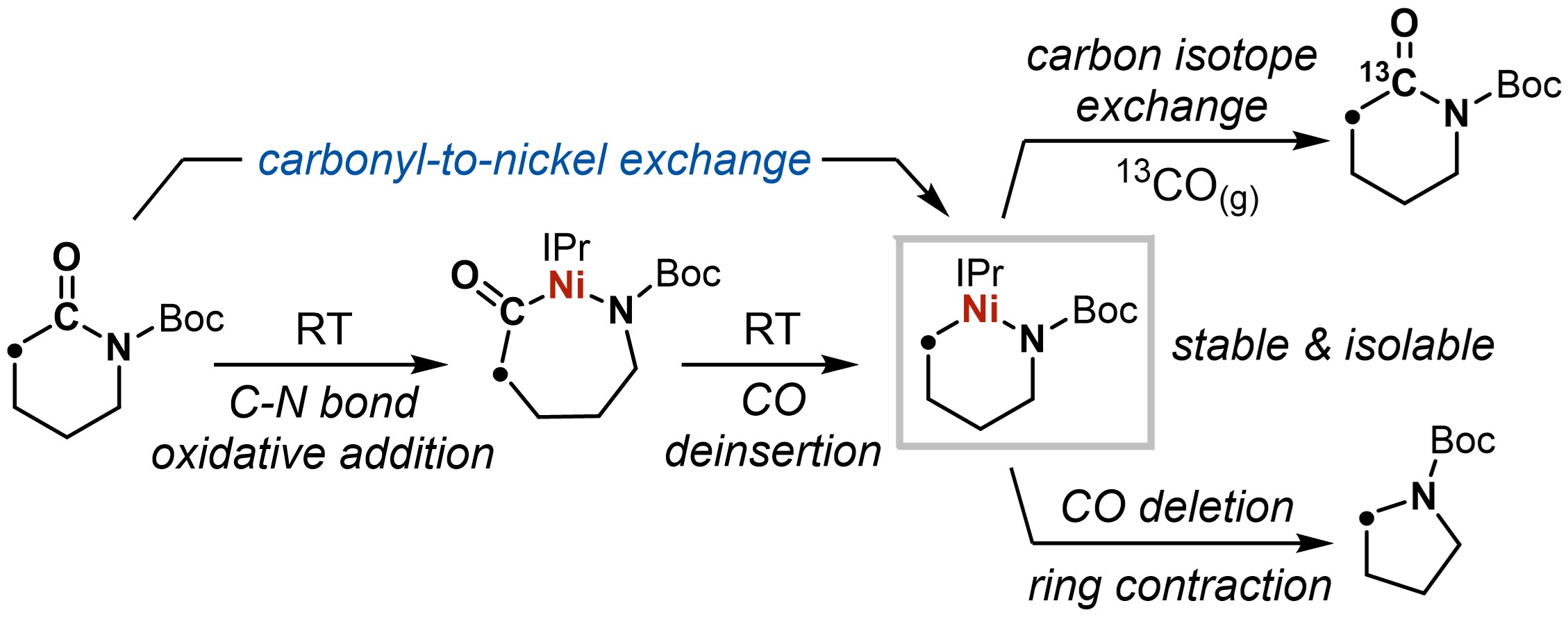
Our laboratory has a fundamental interest in strong bond activation and catalysis with transition metals. In our exploration of molecular shuffling chemistry that aims to exchange the backbone of γ-lactams with olefin donors, we discovered that an (NHC)Ni(0) complex, (IPr)Ni(η6-PhMe), was particularly good at activating Boc-protected lactams of various ring sizes and the reactions even occurred at room temperature, as demonstrated by stoichiometric organometallic studies. More excitingly, CO deinsertion also occurred spontaneously for lactams with ring size greater than six. What we could isolate is the metallacycle product resulting from a net “CO-to-nickel” swap. We were struck by how mild the condition was, and the observation that the “aliphatic” metallacycles – those consist of exclusively or mostly sp3 carbons – are kinetically stable and isolable. This is quite an unusual feature since transition metal alkyl complexes are known to decompose by β-hydride elimination, and in our case that’ll lead to ring-opening.
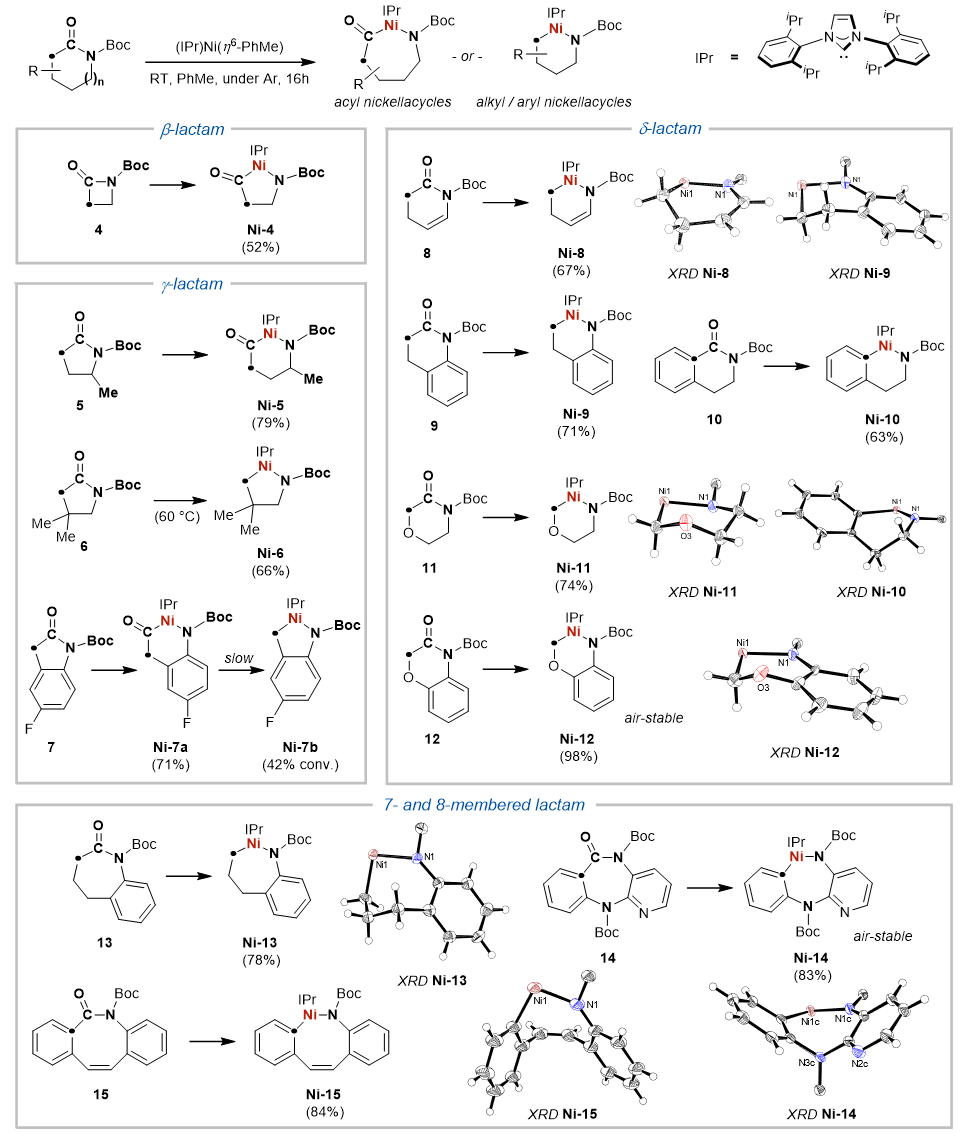
We went on to study this “skeletal metalation” chemistry and it turns out that the reactivity is rather general for unstrained, 6- to 8-membered lactams. The 15 metallacycle products in our study were also isolated and fully characterized, providing definitive evidence for the Ni(0)-mediated C-N bond activation process. We also performed DFT studies to probe the energy landscape, and the reaction was found out to be reversible, which is corroborated by the recovery of initial lactams upon treating the metallacycles with CO(g).
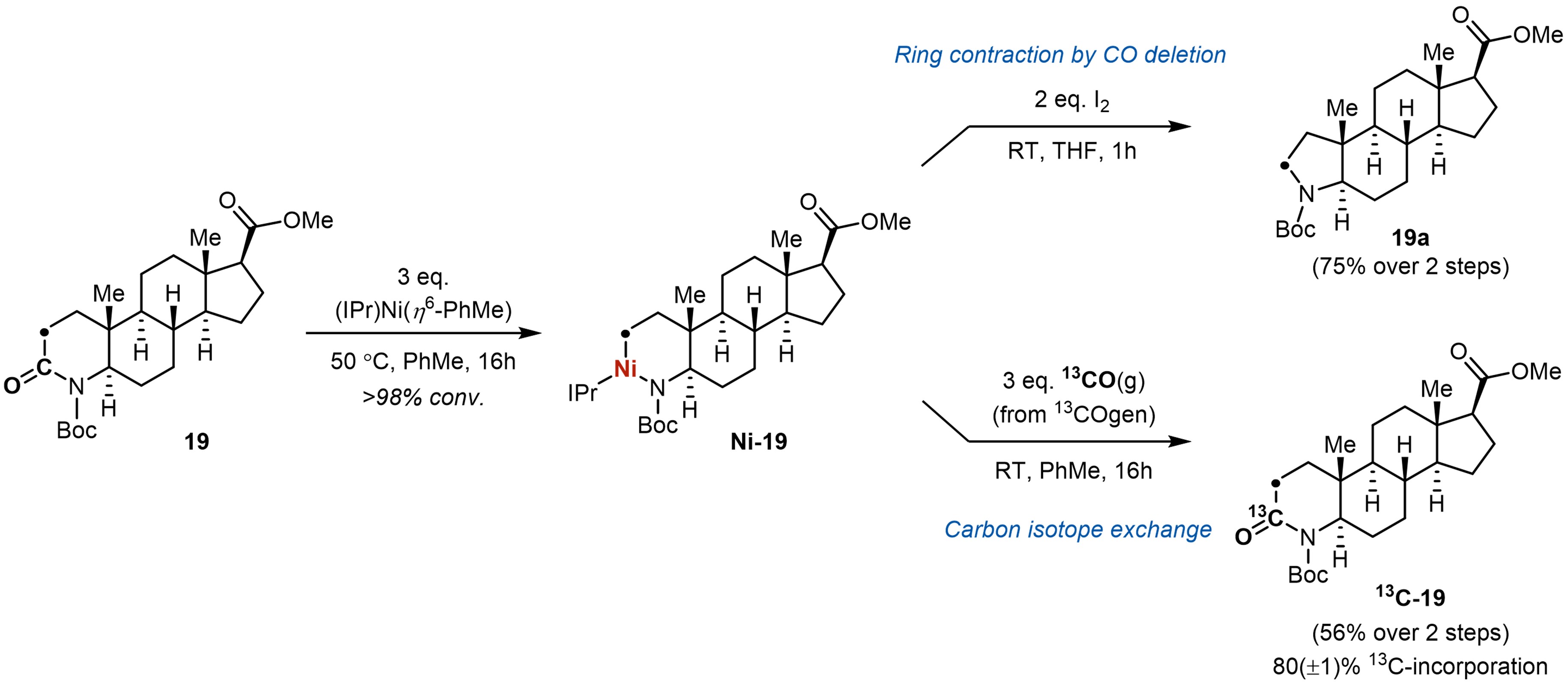
A proof-of-concept molecular editing reaction was demonstrated on an azasteroid drug analogue. We showed that the activation of the lactam ring A was much favored over the C–O bond of a methyl ester or an acidic α C–H bond, highlighting the unique chemoselectivity of the Ni(0) complex (IPr)Ni(η6-PhMe) for cleaving the C(O)–N(Boc) bond. Oxidatively induced reductive elimination of C(sp3)–N bond from Ni(III) successfully afforded the ring contraction product. Notably, accessing the same core structure with a 5-membered pyrrolidine ring A required 6 steps with deliberate ring opening and closing steps, underscoring the high synthetic utility of the metal-carbon exchange strategy. Harnessing the reversible CO deinsertion process of the nickellacycles, a late-stage carbon isotope exchange with 13CO(g) was also successfully achieved, marking the first example of carbon isotope labeling by breaking and forming strong σ-bonds in aliphatic ring structures.
Collectively, these findings demonstrate the high synthetic promise of metal-carbon exchange in molecular editing. For detailed organometallic characterizations and computational studies, please also refer to the Supplementary Information.
References
- Murakami, M., Amii, H. & Ito, Y. Selective activation of carbon–carbon bonds next to a carbonyl group. Nature 370, 540–541 (1994).
- Matsuda, T., Shigeno, M. & Murakami, M. Activation of a cyclobutanone carbon-carbon bond over an aldehyde carbon-hydrogen bond in the rhodium-catalyzed decarbonylation. Chem. Lett. 35, 288–289 (2006).
- Kodama, T., Saito, K. & Tobisu, M. Nickel-catalyzed skeletal transformation of tropone derivatives via C-C bond activation: catalyst-controlled access to diverse ring systems. Chem. Sci. 13, 4922–4929 (2022).
- Luu, Q. H. & Li, J. A C-to-O atom-swapping reaction sequence enabled by Ni-catalyzed decarbonylation of lactones. Chem. Sci. 13, 1095–1100 (2022).
- Jurczyk, J. et al. Single-atom logic for heterocycle editing. Nat. Synth. 1, 352–364 (2022).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in