
Electrochemical energy conversion and storage processes, powered using renewable electricity, provide a sustainable approach to tackling global energy and climate concerns. These processes usually consist of multiple and branched elementary steps involving diverse intermediates and electron/proton transfers. A typical such process is CO2 reduction reaction (CO2RR).
In CO2RR, the judicious stabilization of key intermediates in order to favor a desired pathway is central to the improvement of reaction selectivity. Traditional strategies focus on the control of electrocatalyst materials morphology, grain boundaries, facets, and dopants.
However, the selectivity towards a specific product, especially economically-desirable C2+ products, is still low in neutral media – the best catalyst reported so far shows a Faradaic efficiency (FE) of 60% for ethylene at a partial current density of 7 mA cm-2. This low performance results in a low energy efficiency.
We reported in 2018 that high concentrations of hydroxide ions can mediate the selective production of ethylene, with a record FE of 70% for a Cu catalyst in 10 M KOH. However, large amounts of CO2 are consumed by the alkaline electrolytes to form carbonate – i.e. much CO2 is wasted under these highly alkali conditions.
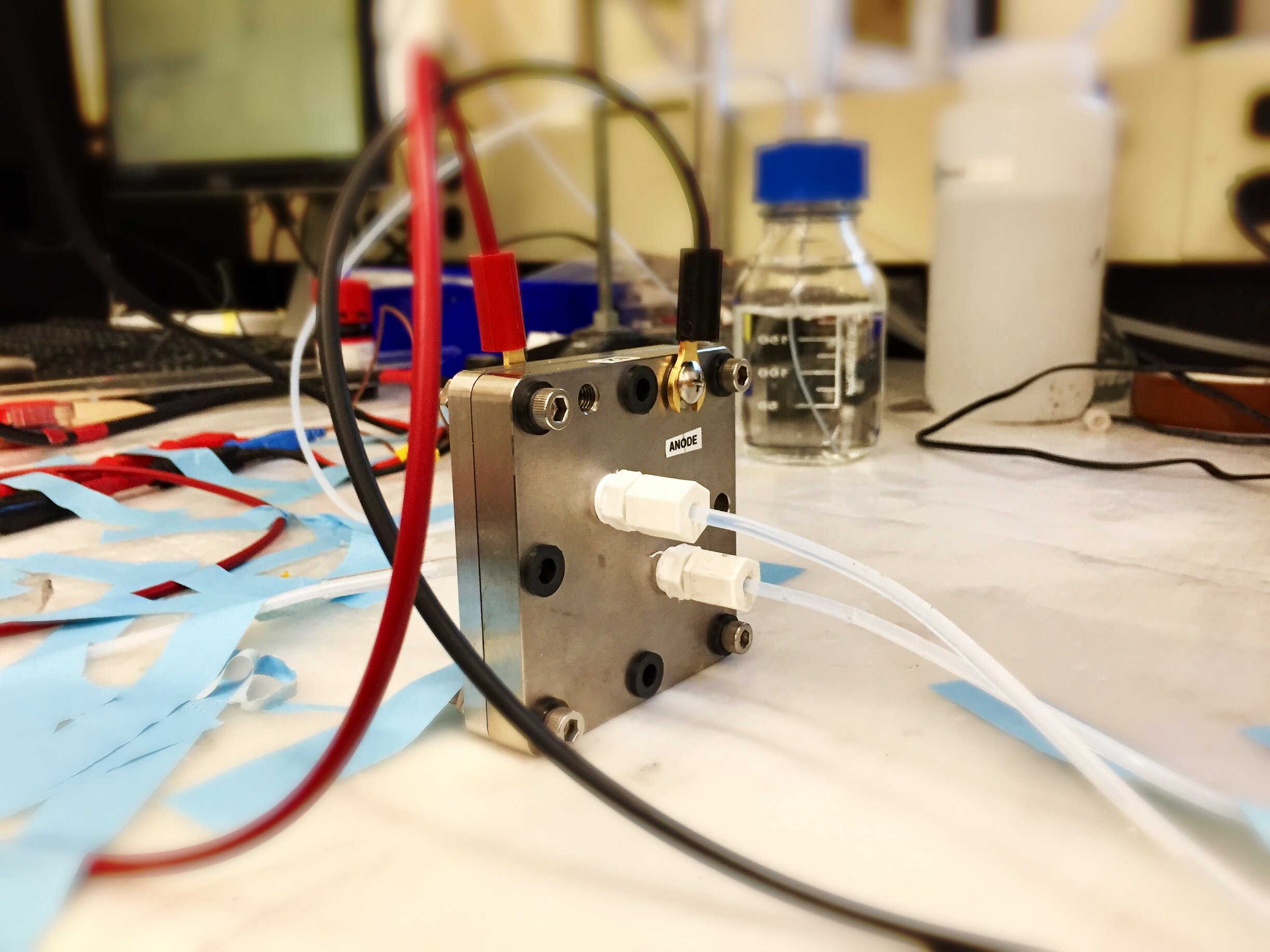
Figure 1. The device that operates CO2-to-ethylene conversion.
More recently, we adopted the approach that – if we could work in neutral media, yet discover how to sustain high selectivity (>70%), and do all this at commercially-relevant current densities (e.g., 300 mA cm-2) – offer a further advance toward the sustainable production of fuels and chemical feedstocks via CO2RR.
Collaborating with the groups of Professor Jonas Peters and Professor Theo Agapie at Caltech, the joint team developed a molecular tuning strategy – the functionalization of the surface of electrocatalysts with organic molecules – that stabilizes intermediates for enhanced CO2RR to ethylene. We investigated a library of molecules, derived via electro-dimerization of arylpyridiniums, with the aid of organic synthesis, operando spectroscopies and theoretical calculations. We found that the adhered molecules stabilize a CO-bound intermediate in an atop bound configuration, favoring reduction to ethylene instead of evolution as gaseous CO.
As a result of this strategy, we achieved CO2RR, in neutral electrolyte, to ethylene, having record performance for this medium: FE of 72% at a partial current density of 230 mA cm-2. The system maintains the high selectivity towards ethylene while operating at high current for 190 hours, delivering a full-cell energy efficiency of 20%. Due to the use of neutral electrolyte, the CO2 is not consumed by the electrolyte: the CO2 utilization efficiency herein is approximately 100%, where in prior energy-efficiency-record-setting reports in alkali media, it was much lower.
The full paper can be found here: https://www.nature.com/articles/s41586-019-1782-2.
Follow the Topic
-
Nature

A weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in