Multi-functional genome-wide CRISPR system for high throughput genotype–phenotype mapping
Published in Bioengineering & Biotechnology

Metabolic engineering has been a key strategy for improving microbial production of bioproducts since the genetic engineering revolution. The typical approach is to select a number of genetic targets for modification (e.g. upregulation of GeneA, downregulation of GeneB) based on their relationship to the pathway of interest, then test the impact of modifying each target. For example, enzymes which produce precursors of the compound of interest may be upregulated, and enzymes which compete for precursors to make their own compounds may be downregulated or eliminated.
That approach has some fundamental limitations though, as only genes already known to have a relationship to the phenotype of interest can be targeted for study, while the majority of genes even in simple organisms such as yeast still have poorly characterized functions. Furthermore, it is harder to predict genetic determinants of traits that arise from interactions of many genes (complex phenotypes, such as growth inhibitor tolerance or protein display) than simpler traits like production of a single compound.
What we have endeavored to create in this work is a CRISPR-based system to trifunctionally perturb the expression of genes in yeast by combining CRISPR activation, CRISPR interference, and homologous recombination-mediated CRISPR deletion (which we called MAGIC). Using a library we designed of 100,000 guide RNAs redundantly targeting each of the 6000 genes in the yeast genome, we were able to create what is to our knowledge one of the most comprehensive mutant libraries in yeast.
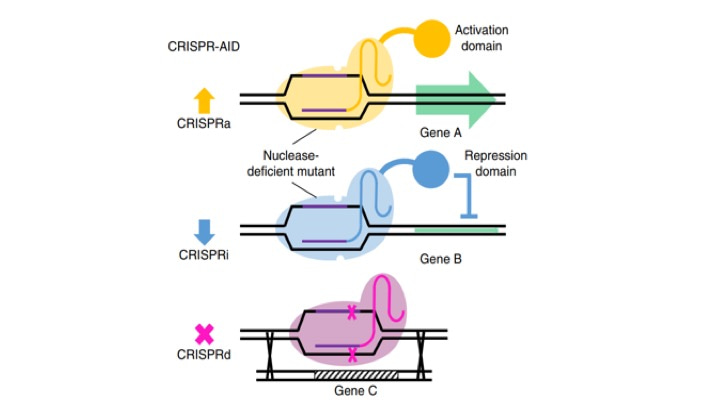
Once the library was created, we could use high-throughput screening such as next-generation sequencing or flow cytometry to identify genetic determinants of various traits, regardless of the complexity of the trait and without any prior knowledge of the genes involved. Furthermore, once one determinant has been identified, the modification can be made permanent and the engineered strain can be iteratively subjected to subsequent additional rounds of screening.
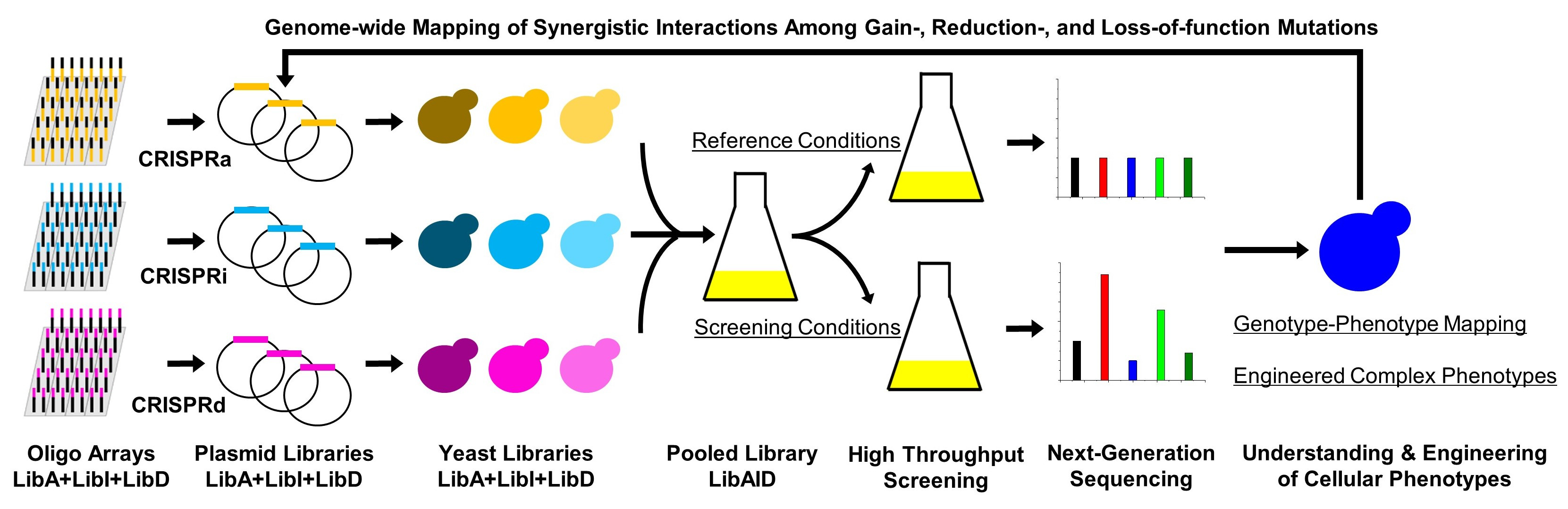
Using this approach, we identified a trifecta of genetic determinants for tolerance of the lignocellulosic growth inhibitor, furfural: interference of SIZ1, activation of NAT1, and interference of PDR1. Together, these genetic modifications enabled creation of a yeast strain that could grow and ferment ethanol in conditions far beyond what killed the wild-type strain. We also showed that these modifications behave in a synergistic manner: the targets discovered in the second and third round of screening confer minimal improvement if any without the presence of the first-round modification. Therefore, these highly effective combinations of targets can only be discovered using genome-scale systems capable of simultaneously maintaining different modes of genetic perturbation, such as MAGIC. In the future, we hope to bring this strategy to the study of a broader array of phenotypes by developing additional high-throughput screening methods such as biosensors, as well as exploring MAGIC in the engineering of more organisms.
This article is based on:
Lian, J., Schultz, C., Cao, M. et al. Multi-functional genome-wide CRISPR system for high throughput genotype–phenotype mapping. Nat Commun 10, 5794 (2019) doi:10.1038/s41467-019-13621-4
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in