Multi-hit TET2 mutations as a new molecular signature in chronic myelomonocytic leukemia
Published in Cancer
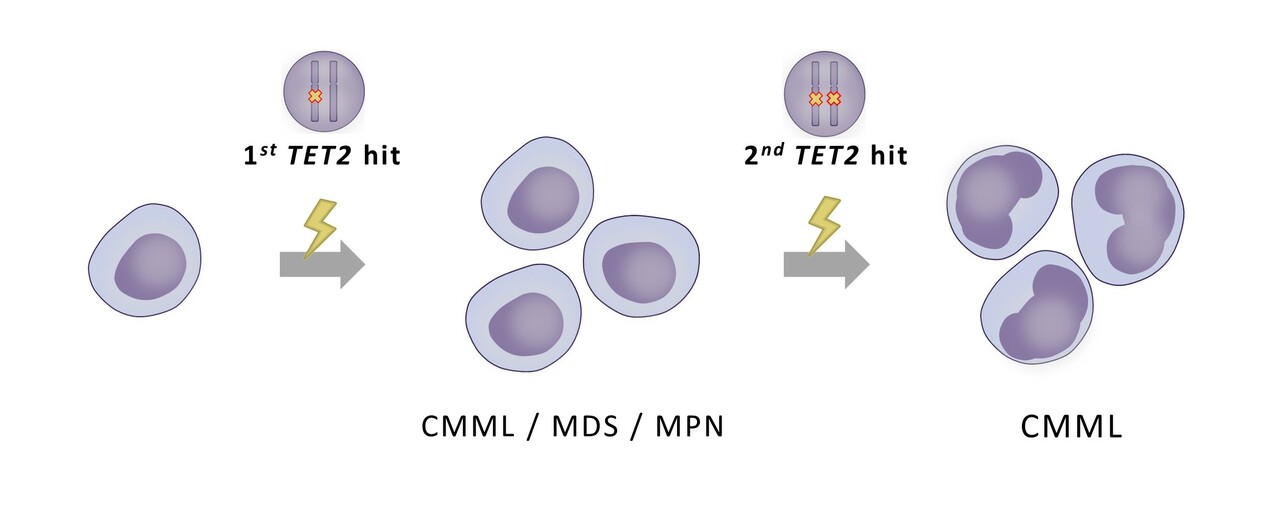
Where did we start?
Chronic myelomonocytic leukemia (CMML) is characterized by sustained peripheral blood monocytosis, therefore persistent absolute and relative peripheral blood monocytosis is required for CMML diagnosis. The upcoming WHO 2022(1) and International Consensus Classification (ICC) 2022(2) have lowered the cutoff for absolute monocytosis from 1×109/L to 0.5×109/L to incorporate oligomonocytic chronic myelomonocytic leukemia (OM-CMML), which are cases with a total monocyte count of 0.5 to 1×109/L(3,4) that show similar clinical, genomic and immunophenotypic features than overt CMML(5).
In previous studies by our group, we assessed the clinical, genomic and immunophenotypic profile (5) and later the clinical outcomes (6) of both overt and OM-CMML. With these studies and other studies as background, we wanted to investigate mutations in TET2 gene, the most prevalent mutated gene in CMML, as a biological clue for overt and OM-CMML diagnosis. Furthermore, we found it very interesting that multiple TET2 mutations were frequently found in the same patient at diagnosis(5,7–10)
We hypothesized that the identification of multiple TET2 mutations could be a molecular signature of overt and OM-CMML as reliable as the widely accepted TET2 and SRSF2 co-mutation(11).
What did we do?
We investigated the presence of multi-hit TET2 mutations (multi-hit TET2 were defined as cases with ≥ 2 TET2 mutations and/or TET2 VAF ≥55%, implying a biallelic alteration due to loss of heterozygosis or 4q24 deletion(10)) in 284 patients with myeloid neoplasia: 54 CMML, 42 OM-CMML, 76 myelodysplastic neoplasms (MDS), 107 Ph-negative myeloproliferative neoplasms (MPN) and 5 MDS/MPN with ring sideroblasts and thrombocytosis (RS-T).
Molecular characterization was performed by next-generation sequencing (NGS) using a custom panel including 25 myeloid-associated genes. The distribution of PB monocyte subsets was assessed in CMML and OM-CMML patients by multiparametric flow cytometry (FC) on whole PB collected on EDTA.
What results have we obtained?
-Our data supports that multi-hit TET2 status would be a new molecular signature for CMML diagnosis. Patients with multi-hit TET2 mutations were identified with similar percentages in OM-CMML (20/42, 47.6%) and CMML (30/54, 55.5%) while they were infrequent in MDS (5/76, 6.6%) and MPN (5/107, 4.7%) (P<0.001).
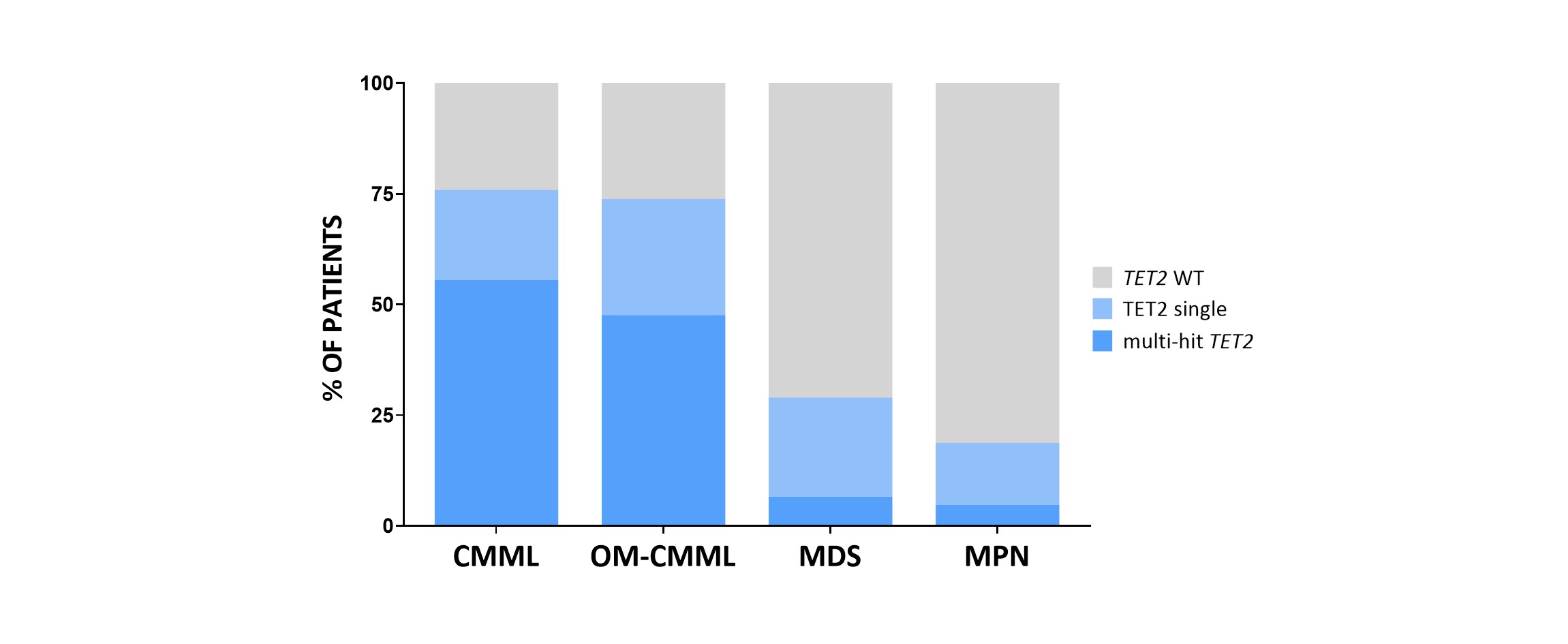 Figure 1. Percentage of patients with TET2 wild type, single TET2 mutation and multi-hit TET2
Figure 1. Percentage of patients with TET2 wild type, single TET2 mutation and multi-hit TET2
-Multi-hit TET2 status shows a higher sensitivity for CMML diagnosis than TET2 and SRSF2 co-mutation. The sensitivity for CMML/OM-CMML diagnosis of multi-hit TET2 was 52.1% (50/96 CMML/OM-CMML) and the specificity was 94.7% (10/188 MDS, MPN, MDS/MPN-RS-T). In contrast, the presence of concomitant mutations in TET2 and SRSF2, the well-accepted molecular signature of CMML, showed a sensitivity of 26.0% (25/96 CMML/OM-CMML) and a specificity of 98.9% (2/188 MDS, MPN, MDS/MPN-RS-T) in our series. Moreover, Youden Index was clearly better for multi-hit TET2 than for TET2 and SRSF2 co-mutation (J = 46.8 vs. J = 24.9, respectively).
-The best strategy is considering both molecular signatures: multi-hit TET2 status and/or TET2 and SRSF2 co-mutation. In our series, the presence of multi-hit TET2 and/or TET2 and SRSF2 co-mutation showed a higher sensitivity (60.4%) maintaining an excellent specificity (94.1%), showing an excellent positive predictive value for overt and OM-CMML diagnosis. Therefore, taking into account both molecular signatures would be a useful strategy for establishing an accurate diagnosis in this setting.
-CMML and OM-CMML show higher TET2 VAF than MDS and MPN patients. OM-CMML and CMML showed similar variant allele frequency (VAF) of their dominant TET2 mutation (1st TET2 hit) (medians 40.8% and 40.3%, respectively). Nonetheless, TET2 VAF was significantly lower in MDS (median 13.34%) than in OM-CMML and CMML (P=0.001 and P=0.003, respectively). A similar trend was observed in MPN patients (median VAF 22.52%) although the difference was only significant for MPN vs. OM-CMML (P =0.047). Of note, CMML and OM-CMML multi-hit TET2 cases presented similar VAF between the 1st TET2 hit and the 2nd TET2 hit, in contrast to MDS and MPN patients, suggesting that both TET2 mutations co-exist and expand in the same clone with biallelic implications.
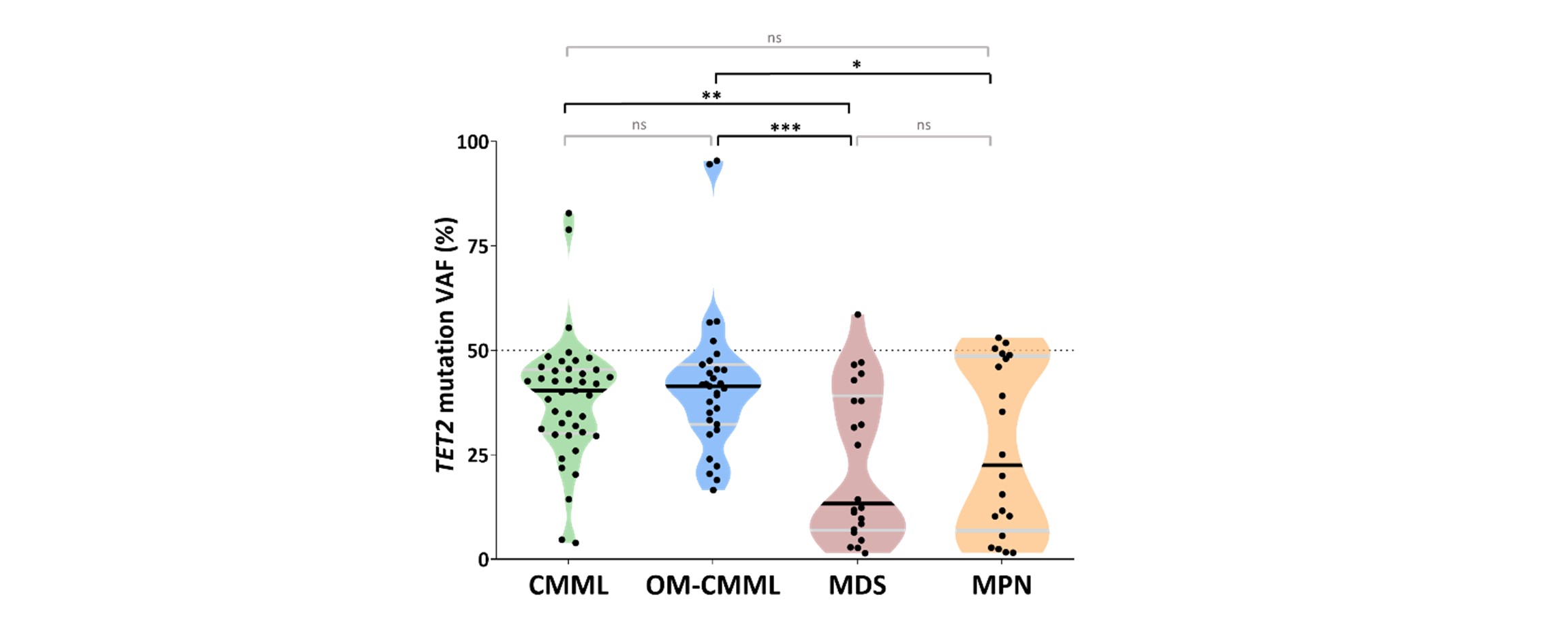 Figure 2. Variant allele frequency (VAF) of TET2 mutations in overt CMML, OM-CMML, MDS and MPN.
Figure 2. Variant allele frequency (VAF) of TET2 mutations in overt CMML, OM-CMML, MDS and MPN.
-Multi-hit TET2 showed differential clinic-biological features. Multi-hit TET2 patients showed a higher percentage of PB and BM monocytes, BM promonocytes and more dysgranulopoiesis than TET2 WT patients. Interestingly, a higher Hb count was identified in multi-hit TET2 cases than in single TET2 cases. Although this finding might allow us to infer that multi-hit TET2 cohort may present patients with more advanced disease, we did not observe significant differences in OS or AML free survival among multi-hit TET2, TET2 single and TET2 WT groups. In TET2 mutated OM-CMML, we observed a higher proportion of patients with a percentage of classical monocytes above 94% (90% mut. vs 40% WT; P=0.001; 81.8%, 94.7% in single and multi-hit TET2, respectively) and CD56 expression on monocytes (70% mut. vs 30% WT; P=0.025; 54.5%, 78.9% in single and multi-hit TET2, respectively).
Which is our main conclusion?
In our series, multi-hit TET2 mutations were frequently identified in overt and OM-CMML patients, while they were infrequent in MDS and MPNs. This, in line with previous studies, supports that multi-hit TET2 status would be a new reliable biological clue for CMML diagnosis and reinforces the consideration of OM-CMML in the biologic spectrum of CMML.
Link to publication: https://www.nature.com/articles/s41375-022-01733-8
Bibliography
- Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leuk 2022. 2022 Jun 22;1–17.
- Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemia: Integrating Morphological, Clinical, and Genomic Data. Blood. 2022 Jun 29;
- Geyer JT, Tam W, Liu Y-C, Chen Z, Wang SA, Bueso-Ramos C, et al. Oligomonocytic chronic myelomonocytic leukemia (chronic myelomonocytic leukemia without absolute monocytosis) displays a similar clinicopathologic and mutational profile to classical chronic myelomonocytic leukemia. Mod Pathol. 2017 Sep 1;30(9):1213–22.
- Valent P, Orazi A, Savona MR, Patnaik MM, Onida F, Loosdrecht AA van de, et al. Proposed diagnostic criteria for classical chronic myelomonocytic leukemia (CMML), CMML variants and pre-CMML conditions. Haematologica. 2019;104(10):1935.
- Calvo X, Garcia-Gisbert N, Parraga I, Gibert J, Florensa L, Andrade-Campos M, et al. Oligomonocytic and overt chronic myelomonocytic leukemia show similar clinical, genomic, and immunophenotypic features. Blood Adv. 2020 Oct 27;4(20):5285–96.
- Calvo X, Roman-Bravo D, Garcia-Gisbert N, Rodriguez-Sevilla JJ, Garcia-Avila S, Florensa L, et al. Outcomes and molecular profile of oligomonocytic CMML support its consideration as the first stage in the CMML continuum. Blood Adv. 2022 Jul 12;6(13):3921–31.
- Itzykson R, Kosmider O, Renneville A, Morabito M, Preudhomme C, Berthon C, et al. Clonal architecture of chronic myelomonocytic leukemias. Blood. 2013 Mar 21;121(12):2186–98.
- Patnaik MM, Zahid MF, Lasho TL, Finke C, Ketterling RL, Gangat N, et al. Number and type of TET2 mutations in chronic myelomonocytic leukemia and their clinical relevance. Blood Cancer J. 2016 Sep;6(9):e472.
- Coltro G, Mangaonkar AA, Lasho TL, Finke CM, Pophali P, Carr R, et al. Clinical, molecular, and prognostic correlates of number, type, and functional localization of TET2 mutations in chronic myelomonocytic leukemia (CMML)-a study of 1084 patients. Leukemia. 2020 May 1;34(5):1407–21.
- Awada H, Nagata Y, Goyal A, Asad MF, Patel B, Hirsch CM, et al. Invariant phenotype and molecular association of biallelic TET2 mutant myeloid neoplasia. Blood Adv. 2019 Feb 12;3(3):339–49.
- Palomo L, Meggendorfer M, Hutter S, Twardziok S, Ademà V, Fuhrmann I, et al. Molecular landscape and clonal architecture of adult myelodysplastic/myeloproliferative neoplasms. Blood. 2020 Oct 15;136(16):1851–62.
Follow the Topic
-
Leukemia

This journal publishes high quality, peer reviewed research that covers all aspects of the research and treatment of leukemia and allied diseases. Topics of interest include oncogenes, growth factors, stem cells, leukemia genomics, cell cycle, signal transduction and molecular targets for therapy.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in