Background and Rationale
Cancer growth and anti-cancer treatments generate detrimental adaptations in patients, frequently resulting in paraneoplastic syndromes, among which cancer cachexia (CC) is the most relevant and impactful. CC is characterized by systemic inflammation and metabolic stress in several organs, resulting in impaired tissue function, reduced tolerance to chemotherapy and poor immune response, all contributing to impaired quality of life and reduced survival1. In the oncology practice, most if not all of the attention is focused on cancer-directed therapeutics, frequently disregarding the host status and missing the opportunity to treat the cancer and the associated host syndromes as a unique disease. Clinically, CC is diagnosed as the involuntary loss of body weight in the last 6 months and/or the occurrence of sarcopenia (i.e. loss of muscle mass)1. This phenotype results mainly from protein hypercatabolism and reflects the altered energy metabolism consequent to mitochondrial dysfunction2. However, only few preclinical studies have characterized energy metabolism alterations in cancer hosts and demonstrated the effectiveness of targeting such alterations in counteracting CC3.
In the current research we targeted energy metabolism to find novel anti-cachexia treatment options. The identification of the specific target, i.e. NAD⁺ metabolism, occurred thanks to our collaborator Juha Hulmi from Jyväskylä, Finland, whose proteomic analysis in the skeletal muscle of C26-bearing mice showed that impaired mitochondrial oxidation was coupled with NAD⁺ shortage4. Since NAD⁺ is critical for mitochondrial redox reactions, NAD⁺ loss may explain the energy failure occurring in the muscle of cachectic animals, similarly than in primary mitochondrial myopathy, where NAD⁺ boosting with vitamin B3 niacin counteracted muscle dysmetabolism, as shown by the pioneering work of Eija Pirinen in Helsinki, Finland5.
Novelty
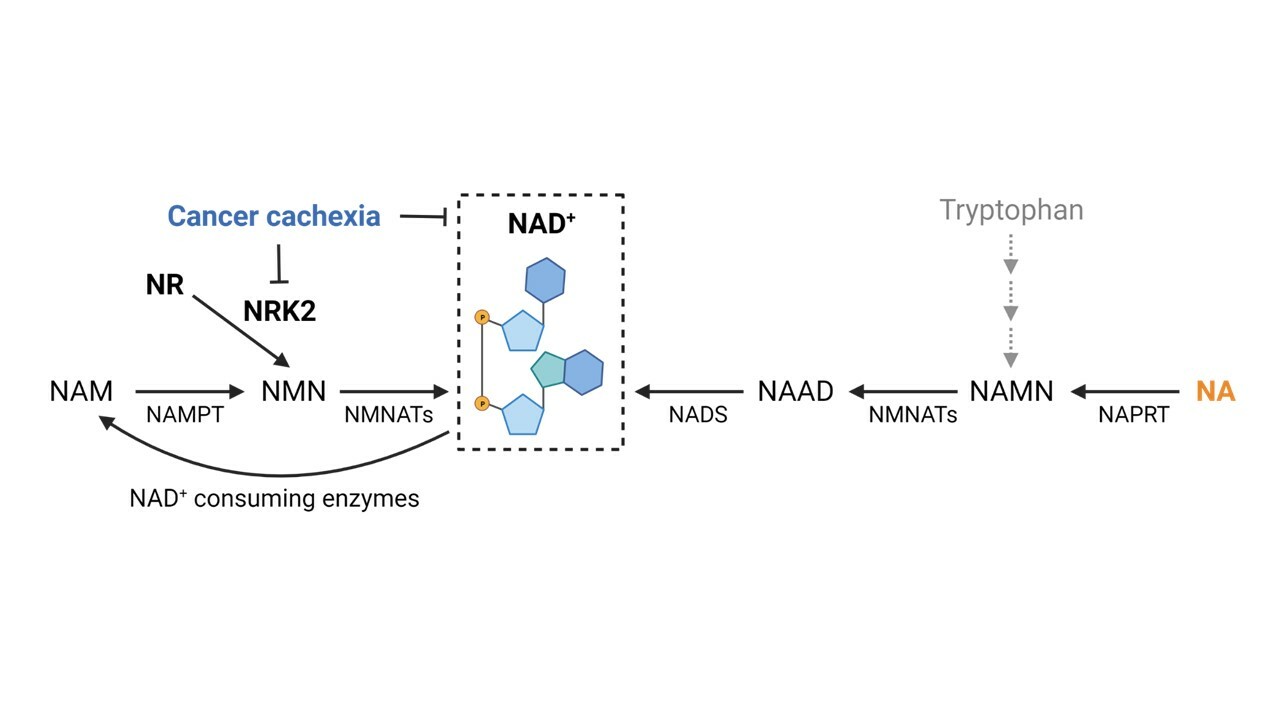
We decided to characterize NAD⁺ metabolism in the context of CC and to test the effectiveness of NAD⁺ repletion in tumor bearing-mice, adopting improved and more clinically relevant mouse models of cancer cachexia that the junior post-doctoral fellow Marc Beltrà was developing in Torino, Italy. The intervention study was preceded by the work of PhD student Noora Pöllänen (Helsinki, Finland), whose screening of NAD⁺ metabolism disturbances in these different CC models confirmed that muscle NAD⁺ loss is a common trait in experimental cachexia triggered by intestinal and pancreatic cancers, especially in severe conditions. Beyond NAD⁺ deficiency, the transcript of the NAD⁺ biosynthetic enzyme nicotinamide riboside kinase 2 (Nrk2), metabolizing nicotinamide riboside (NR) towards NAD⁺, was consistently reduced in all the models examined (Figure 1). In the attempt to extend to and validate this observation in human cancer cachexia, the group of the assistant professor Roberta Sartori in Padova, Italy, joined the consortium and proved the occurrence of NRK2 loss and impaired energy metabolism in the skeletal muscle of colorectal and pancreatic cancer patients, independently from the cachexia status.
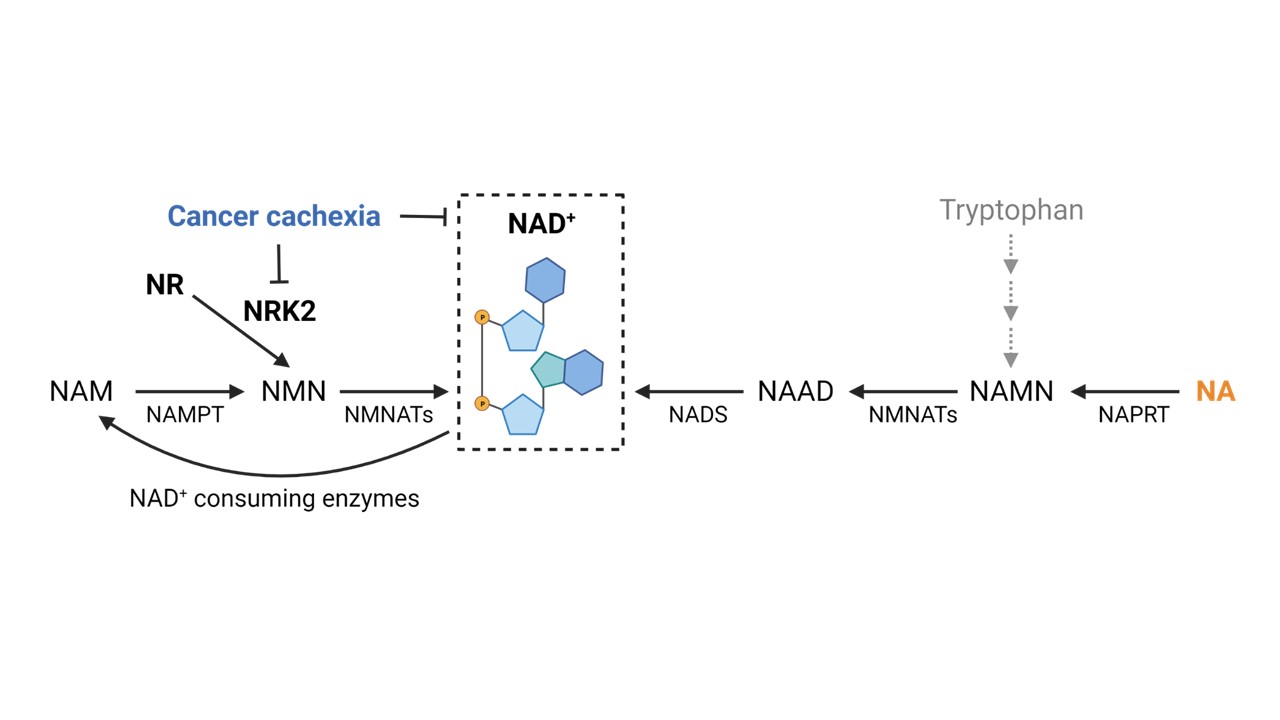
Figure 1. Nicotinamide riboside kinase 2 (NRK2) repression in cancer cachexia. NR: nicotinamide riboside; NA: niacin; NAAD: nicotinic acid adenine dinucleotide; NADS: NAD synthetase; NAM: nicotinamide; NAMN: nicotinate mononucleotide; NAMPT: nicotinamide phosphoribosyltransferase; NAPRT: nicotinic acid phosphoribosyltransferase; NMN: nicotinamide mononucleotide; NMNATs: nicotinamide mononucleotide adenylyl transferases.
Considering the repression of Nrk2 likely impairing nicotinamide riboside (NR) ability to rescue NAD⁺, we chose to test the effectiveness of niacin (NA) in boosting NAD⁺ levels in tumor bearing-mice. Both acute and chronic models of cachexia induced by intestinal cancers were used (Figure 2). Niacin rescued the muscle NAD⁺ deficiency observed in the acute CC, and prompted mitochondrial biogenesis, partially counteracting muscle wasting and energy metabolism alterations in acute and chronic CC. Moreover, NAD⁺ loss was also observed in the liver in both acute and chronic experimental CC, whereas niacin effectively prevented the depletion, showing that NAD⁺ metabolism disturbances are systemic and not restricted to the skeletal muscle of tumor hosts.
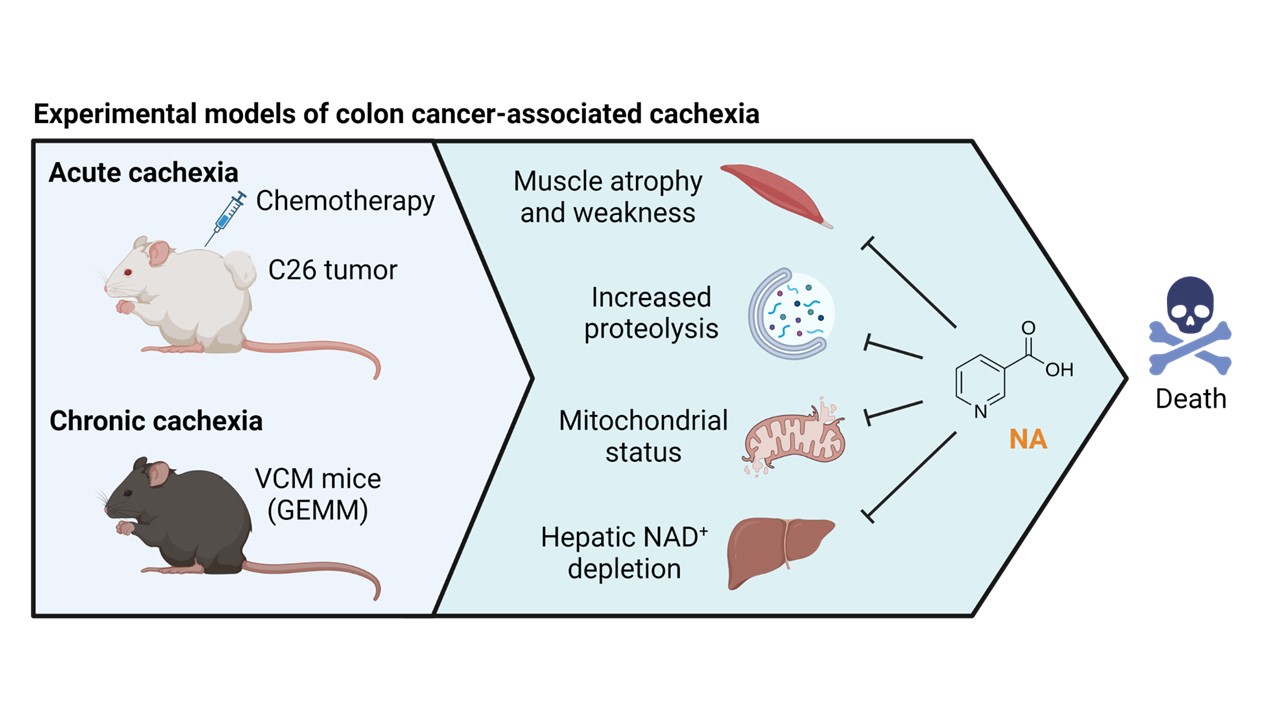
Figure 2. NAD⁺ repletion counteracts experimental cancer cachexia. Models used (left) and impact of niacin (NA) administration (right). GEMM: genetically engineered mouse model; VCM: Villin-Cre/Msh2loxP/loxP.
Take home message and new directions
Our evidence emphasizes Nrk2 and NAD⁺ loss as common features of CC. NAD⁺ replenishment with niacin rescues energy homeostasis in tumor-bearing animals, counteracting CC. Considering that niacin is inexpensive and has been safely used for treating for example hypercholesterolemia in humans, we propose its use for the management of cancer patients presenting with impaired systemic energy metabolism. Targeting the metabolic imbalance in the host will potentially revert the vicious cycle of cachexia and therapy unresponsiveness, improving cancer patient survival and quality of life.
NRK2 loss and the associated metabolic impairment in preclinical and clinical CC provide also new insights that may reveal cancer-associated metabolic alterations preceding the onset of overt cachexia and even turn into biomarkers for CC. In the future, circulating levels of NAD⁺ may be rapidly measured for guiding the choice of supportive anti-cachexia interventions, including niacin.
References
- Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 (2011).
- Rohm,M., Zeigerer, A.,Machado, J. & Herzig, S. Energy metabolism in cachexia. EMBO Rep. 20, e47258 (2019).
- Huot, J.R. et al. Targeting Mitochondria and Oxidative Stress in Cancer- and Chemotherapy-Induced Muscle Wasting. Antioxid Redox Signal. 38, 352-370 (2023).
- Hulmi, J. J. et al. Muscle NAD+ depletion and Serpina3n as molecular determinants of murine cancer cachexia—the effects of blocking myostatin and activins. Mol. Metab. 41, 101046 (2020).
- Pirinen, E. et al. Niacin Cures Systemic NAD+ Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy. Cell Metab. 31, 1078–1090.e5 (2020).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in