Nanofluidic Sensing
Published in Earth & Environment, Electrical & Electronic Engineering, and Materials
Nanoconfined water has unique physical and chemical properties, which play key roles in various applications such as sensing, filtration, energy conversion, and catalysis. However, conventional control of confined water transport by varying the pH, temperature, or ionic species, suffers from low stability and poor controllability. In contrast, the emerging electrical control strategy overcomes these drawbacks and has attracted extensive attention.1,2 In 2018, an interesting electrically controlled water permeation was reported in graphene oxide membranes in which the confined water permeation rate was decreased following the increase of voltage until complete blocking at ~ 2.0 V.1 However, two remaining challenges need to be addressed: (1) to realize the precise and fast switch between speeding up and slowing down the water permeation rate by voltage control in a membrane. (2) to make the effective voltage-control range lower than the theoretical water-splitting voltage (1.23 V).
In our newly published work,3 we chose a two-dimensional (2D) transition metal carbide (Ti3C2 MXene) with unique layered structures as the research template of water molecules transport in confined spaces and proposed the concept of combining cations and electric factors to address the above key challenges. This strategy enables to rapidly regulate the permeation rate of controlled water on the metal cation-intercalated Ti3C2 (K-Ti3C2 and Li-Ti3C2) membranes, which is attributed to the equilibrium transformation of confined water between three structural types caused by electric fields and cations: monomer ⇋ dimer ⇋ cluste. In 0 ~ 0.9 V, we found that an orderly denser arrangement of confined water caused by low currents that induced the polarization of water molecules results in a significant increase in water permeation rate with increasing voltage. Subsequently, the reduced free energy barrier of metal cations weakened the suppression of current, leading to a sudden change in current and a sharp decrease in permeability at ~ 0.9 V. The reason is that the high current drove the dissociation and agglomeration of confined hydrolysis into macromolecular clusters in 2D channels, hindering the flow of water molecules. Based on the findings, we developed a high-performance humidity sensor by simultaneously optimizing the response and recovery speeds through electric manipulation.

Fig. 1 | Dynamics and structural evolution of water molecules in 2D nanoconfined channels under electrical manipulation.
Firstly, the membrane consists of a Ti3C2 MXene permeation membrane and bilateral porous Au electrodes (Fig. 1A). We fabricated the membrane by assembling a porous gold film with multilayer MXene layer by vacuum-assisted filtration. Subsequently, we measured the water permeability of MXene membranes using the typical gravimetric method to evaluate the effect of the electric field on the movement behavior of water molecules (Fig. 1B). When the voltage was applied to the membranes within 0 ~ 0.9 V, the water permeation rates in the three membranes increased with different slopes (K-Ti3C2 ≈ Li-Ti3C2 >> Ti3C2) following the voltage rise. Notably, the permeation performance of the K-Ti3C2 and Li-Ti3C2 membranes exhibited strong voltage sensitivity and reached the maximum permeation rates of 24.12 and 22.87 g h-1 cm-2 at ~ 0.9 V. Unexpectedly, the permeation rates of K-Ti3C2 and Li-Ti3C2 membranes started dropping sharply from 0.9 V and then quickly returned to the initial voltage-free value. Ultimately, the water permeation through the K-Ti3C2 and Li-Ti3C2 membranes further decreased at 10 V. The permeation rates of water were only 0.84 and 2.74 g h-1 cm-2, respectively. Herein, we observed the permeation rates and currents of K-Ti3C2 and Li-Ti3C2 undergo a sudden change almost simultaneously, thus we selected K+ ion-intercalated and original Ti3C2 for further study.
To explore the dynamics and structure of water molecules for electrical control in the 2D channel, we recorded the infrared spectra of the samples under different voltages at 100% RH, and then we convoluted the water stretching vibrational bands into five distinct components by Gaussian fitting (Fig. 1C). At voltages < 0.9 V, we observed that both the bending vibrational peaks and stretching vibrational bands of the confined water in K-Ti3C2 increase with voltage rise, indicating a potential alteration in the amount of water within the nanoconfined space. Subsequently, through in situ XRD and contact angle measurements, we ruled out the possibility of changes in the number of water molecules caused by changes in effective channel size or surface-wetting ability. Ultimately, the phenomenon was attributed to the directional polarization and electronic polarization of the confined water caused by voltage. Fig. 1D and E depict the transition states of water molecules from random to ordered. At 0 potential, the spatial utilization efficiency of K-Ti3C2 in 2D channels is not high. At this point, the water molecules are entrapped, and the disordered water molecules lead to more spatial gaps in the channel. With the increase of voltage, some hydrogen bonds are bent and locally broken due to the polarization effect, which eventually leads to a regular distribution of electrical confined water in the channel. The densification of water molecules in the electrical channel is greatly improved compared to the disordered water molecules.
We then conducted an in-depth analysis for the unexpected sudden change when the voltage > 0.9 V. Firstly, we eliminated the influence of the Joule heating effect on the water permeation rate by recording the surface temperature of the K-Ti3C2 membrane near the current mutation point. The Gaussian-fitting spectra reveal (Fig. 1C) that the number of water clusters significantly increases to ~ 38% of the amount of confined water and the size of the cluster is increased (3200 cm-1) at 1 V, while the intensity of the water stretching bands has no significant change. With the increase in voltage, the number of water clusters once increased to ~ 42%. The strong intermolecular electrostatic attraction induces spontaneous aggregation of the surrounding water molecules to form strong associative molecular chains, thus hindering the transportation of confined water in the nanoconfined space. Subsequently, AIMD simulations further confirmed that the flow rate of water molecules decreases with increasing ion concentration, which is consistent with the infrared spectroscopy results. Therefore, the confined water permeation under heavy current can be mainly attributed to the large-sized hydrated ion clusters that block the 2D nanochannels.
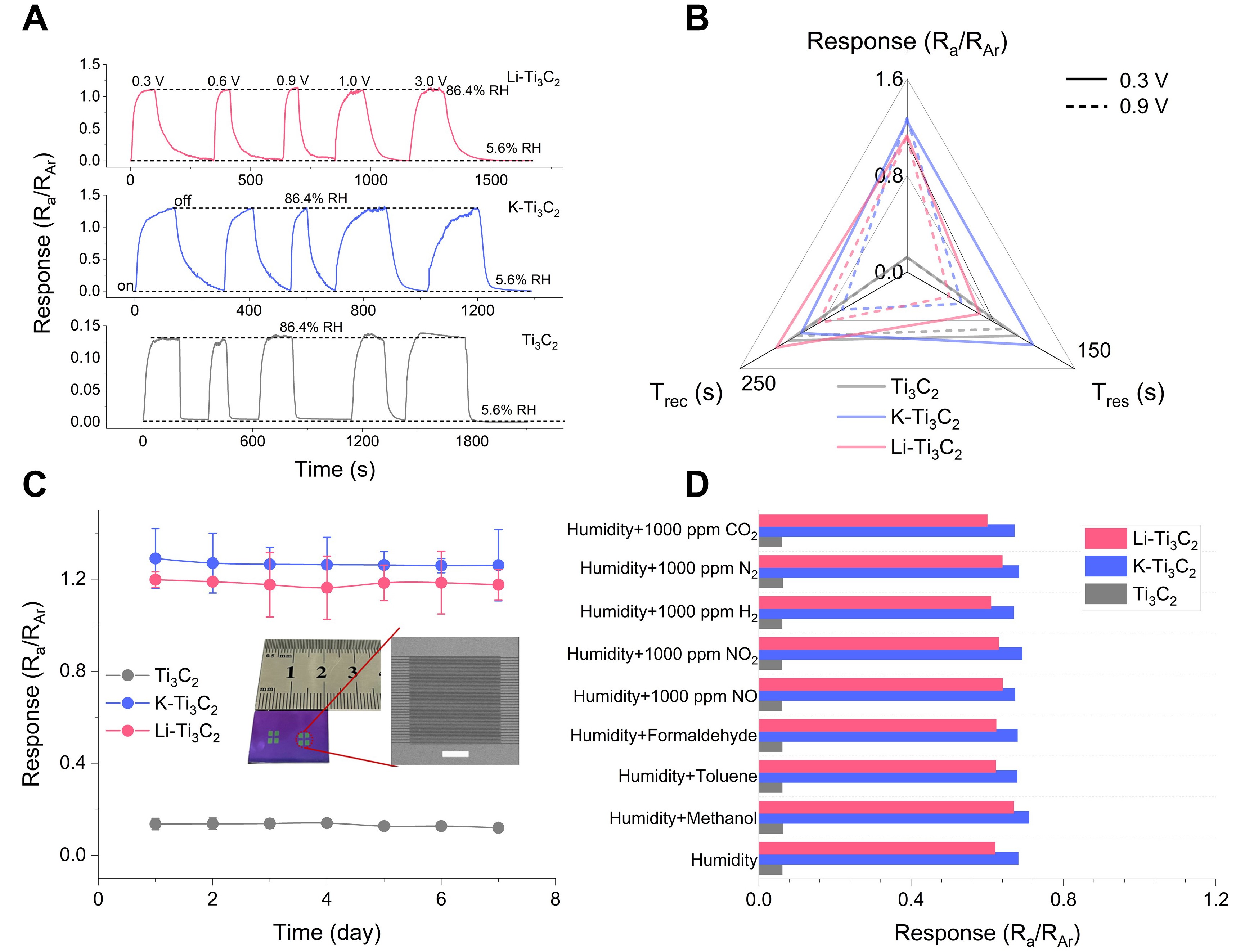
Fig. 2 | Nanofluid humidity sensing performance with electrical control.
Inspired by the anomalous gating phenomenon, the samples were evaluated as sensitive materials for humidity detection to explore the nanofluidic sensing paradigm. The optical image in Fig. 2C shows a micro-scale sensing substrate based on our photolithography fabrication. Briefly, the manufacturing process involves the use of photolithography technology to create interdigital electrode patterns at the micron level on silicon wafers. Subsequently, non-conductive resin is transformed into glass carbon with desirable conductivity by subjecting it to high-temperature pyrolysis. The experiment results indicate that the K-Ti3C2 (1.27) and Li-Ti3C2 (1.12) have significant improvements in humidity responses, which are about 10 and 8 times higher than Ti3C2, respectively (Fig. 2A). This can be attributed to the introduction of metal cations which enlarged the effective interlayer spaces, allowing more water molecules to enter into the channel interior. Noteworthy that when the voltage increases from 0.3 to 0.9 V, the response and recovery speed of K-Ti3C2 (Response time: 113→49 s; Recovery time: 158→97 s) and Li-Ti3C2 (Response time: 65→38 s; Recovery time: 195→132 s) are greatly improved (Fig. 2B). Therefore, electric and ionic manipulations are promising methods for developing nanofluidic sensing applications.
References
- Zhou, K. G. et al.Electrically controlled water permeation through graphene oxide membranes. Nature559, 236–240 (2018).
- Mouterde, T. et al.Molecular streaming and its voltage control in ångström-scale channels. Nature567, 87–90 (2019).
- Chu, T.; Zhou, Z.; Tian, P.; Yu, T.; Lian, C.; Zhang, B.; Xuan, F.-Z. Nanofluidic Sensing Inspired by the Anomalous Water Dynamics in Electrical Angstrom-scale Channels. Nature Communications2024, 15, 7329.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in