Nanoscale Reference and Test Materials for the Validation of Characterization Methods for Engineered Nanomaterials – Current State, Limitations and Needs
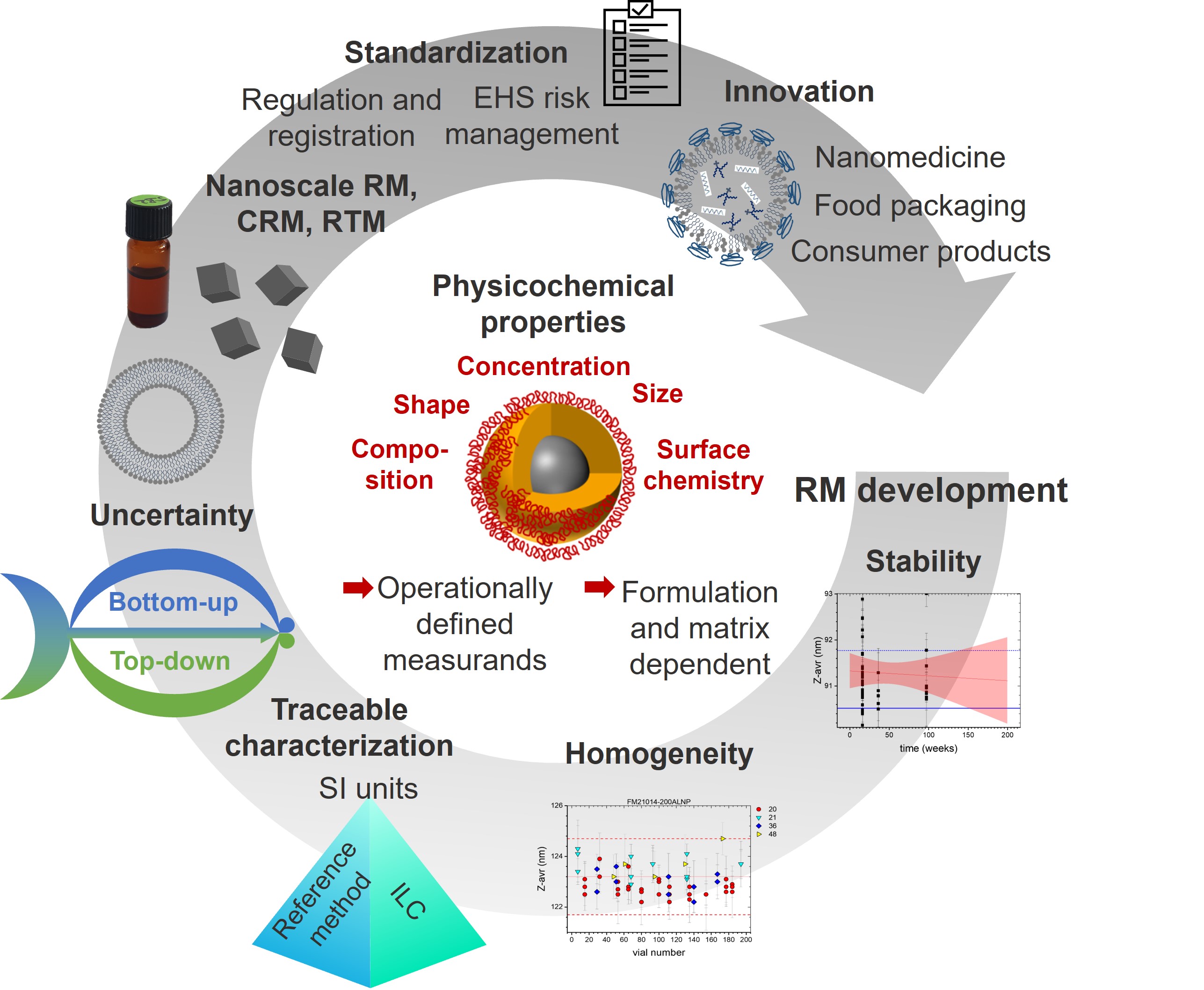
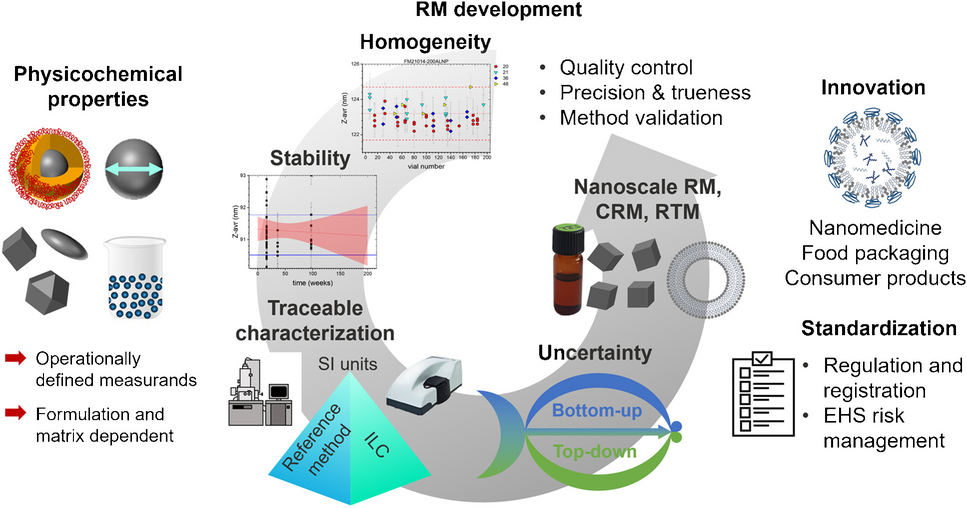
Despite the increasing use of NMs, adequate characterization data are often lacking, hampering the comparability of measurements, value of toxicity studies, and safe and sustainable NM design efforts. The stringent requirements for NM characterization require the availability of validated measurement methods for a range of properties. This task is facilitated by suitable reference materials (RMs) providing benchmark values that can be used to test and validate instrument performance, establish and validate measurement methods, verify results, and assess laboratory competence. Such activities are particularly important in heavily regulated areas such as medical diagnostics and therapies. These needs encouraged us to review the current state, gaps, and future needs for RM production, thereby also providing information on the metrological hierarchy and characterization of different types of RMs, RM certification, and an overview of existing nanoscale RM, see also Figure 1, highlighting existing nanoscale (certified) reference materials ((C)RMs) and reference test materials (RTMs) as well as current gaps.
Reference Material Definitions
The gold standard for RMs is a certified reference material (CRM) which is defined by ISO as a material characterized by a metrologically valid procedure, accompanied by a certificate specifying the property value along with a statement of metrological traceability. Metrological traceability requires that the measurement result can be related to a reference through a documented, unbroken chain of calibrations to an SI unit, with each step contributing to the measurement uncertainty. An RM is defined as a material that is sufficiently homogeneous and stable with respect to one or more specified properties and has been established to be fit for the intended scope of a measurement process. Neither an uncertainty estimation nor traceability is required, although detailed reports and reference data are typically provided. Both CRMs and RMs often include additional data on other parameters that may be useful to the end user, despite the lack of traceability or uncertainty. While some CRMs are available from industrial sources, they do not always provide a full description of metrological traceability as required by ISO standards. Given the wide diversity of NMs, there is a clear need not only for CRMs and RMs but also for test or quality control materials. These materials, which have often been developed and used for interlaboratory comparisons (ILCs), are stable and homogeneous for specific application-relevant properties.
Certification of Reference Materials
Resource-intensive RM certification, which is most often accomplished by a national metrology institute as part of their mandate to support standardization, includes several steps. First, a material must be selected that is available in adequate supply and sufficiently stable for its intended purpose. Next, the stability and homogeneity of the material must be assessed, as shown in Figure 1, followed by a plan for characterizing the properties to be certified using a metrologically valid procedure. This process is exemplarily highlighted for two recent CRMs that address current challenges in producing application relevant materials: iron oxide nanoparticles of cubic shape, released by BAM, and liposomes and lipid-based nanoparticles from NRC, which address nanomedicine needs.
Current Gaps and Future Challenges and Needs
Available CRMs and RMs are mostly spherical nanoparticles with relatively monodisperse size distributions and certified or reference values for size or specific surface area. They also often have informative data for other properties. To highlight current gaps, we first summarize over 30 entries for CRMs and RMs available for NMIs and other government organizations in this review. One notable gap is the lack of materials with non-spherical shapes as recently addressed by the cubic iron oxide nanoparticles from BAM, and/or high polydispersity, both of which are critical for particle size measurements and the determination of number concentration. There are also almost no materials available in complex matrices. Most available RMs in simple matrices are ill-suited for characterizing NMs in real-world environmental or biomedical samples or in consumer products. There are also relatively few methods for which certified values are available for measurands other than particle size distribution or specific surface area. Therefore, large metrology projects and recent RM production in different countries are beginning to address these pressing needs. Particularly relevant are RMs for particle number concentration for new EU legislative requirements, RMs to identify and quantify FGs on NM for NM risk assessment, and lipid based RMs for nanomedicine needs.
Important Areas for Future Work
The nano applications community must prioritize measurands for which RMs are urgently needed, considering the expected user base. For example, the development of multi-measurand RMs can be useful for a broader range of applications. The utility of RMs can be increased by ensuring that the materials are designed for specific applications, e.g., deposition on substrates for microscopy to eliminate sample preparation issues. Increased availability of materials that have been well-characterized from ILCs will also facilitate method development, even in the absence of traceability. Also, a more concerted effort from the nano community is needed to make reliable and validated data on characterization of NMs readily available in standard databases. This should be coupled with an effort to ensure a greater awareness of the availability of validated method protocols, RMs and standards among scientists who develop applications, particularly in industry, and in general method validation. Although progress is still needed to establish standard methods and approaches for all applications and risk-relevant properties of NMs, these improvements will present a significant step towards the successful utilization of NMs in fields ranging from optical and sensor technologies to the health and consumer product sectors.
.
Follow the Topic
-
Analytical and Bioanalytical Chemistry

Analytical and Bioanalytical Chemistry (ABC) is the only journal with global visibility and the mission to rapidly publish excellent and high-impact contributions on fundamental and applied topics of (bio-)analytical research that is supported by a large group of learned societies around the world.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in