Nanosensor Detection of Reactive Oxygen and Nitrogen Species Leakage in Frustrated Phagocytosis of Nanofibers
Published in Chemistry, Earth & Environment, and Materials
Our team of researchers from the Wuhan University (China), École Normale Supérieure and CNRS in Paris (France), Xiamen University (China) and Tianjin University (China) has recently published an article in Nature Nanotechnology, which showcases our work in reactive oxygen and nitrogen species (ROS/RNS) leakage during frustrated phagocytosis of glass nanofibres characterized by nanosensor (Fig. 1a). This paper is the culmination of several years of work on a project that involved team members with a wide range of scientific backgrounds after we could establish through a series of works that ROS/RNS homeostasis is an intrinsic characteristic of phagolysosomes when macrophages undergo phagocytotic actions. It demonstrates for the first time that the existence of an intense prolonged release of ROS/RNS by single macrophages at their phagocytic cups and these continued massive ROS/RNS leakage could damage peripheral cells and eventually translate into lung injury.
Prof. Christian Amatore and I have been very close friends and active collaborators for many years, and we have joined our expertise on many studies in nanoelectrode-based single-cell analysis. Two years ago, in parallel with our scientific discussions, we became fascinated by the web photos of macrophages engulfing bacilli with long aspect ratios such as Bacillus anthracis. These images inspired us to speculate that phagocytosis of inert nanofibrous nanomaterials with long aspect ratios, which are difficult for macrophages to degrade or encapsulate, should lead to aborted phagocytosis. Indeed, unlike pathogens that are easily engulfed inside sealed phagolysosomes of macrophages, fibrous nanomaterials are too long and stable to degrade in pieces gradually, which causes frustrated phagocytosis1. Over the past decades, several studies using ROS/RNS staining or extended in vivo experiments with fibrous nanomaterials for environmental exposure, including carbon nanotubes and asbestos, have supported this hypothesis2. However, neither the chemical nature of the leaking reactive species nor the spatiotemporal characteristics of their distributions or their fluxes intensities could be revealed due to the lack of suitable precise analytical sensors. Nonetheless, these characteristics are key factors related to these purported ROS/RNS fluxes to medical pulmonary issues.
Not long before our idea took shape, Miss Yu-Ting developed a special type of Pt-black nanowire sensor (SiC@Pt NW sensor), which possesses the unique ability to identify and quantitatively monitor the four primary ROS/RNS (ONOO-, H2O2, NO and NO2-)3. Therefore, Christian and I immediately encouraged her to utilize this nanosensor to track ROS/RNS during frustrated phagocytosis.
Even if we had in mind asbestos at the origin of this work owing to the established health issues associated to its nanofibres, we selected glass nanofibre, a chemically inert substance widely used but with contentious biotoxicity, for our research because any question related to the chemical properties of the nanofibres surfaces could be de facto disregarded so we could focus on the effects connected with the geometrical characteristics. However, commercially available glass fibres have irregular diameters and are difficult to disperse in aqueous systems. Coincidentally, Prof. Pingqing Fu sent us a collection of atmospheric particulate matter samples for studying the oxidative stress of macrophages on atmospheric nanoparticles. Possibly due to mishandling, the nanoparticles sample included numerous nanofibres from the quartz filter membrane used to collect particulate matter. Interestingly, we observed frustrated phagocytosis with the microscope when we incubated this cluster of "unsuccessful" samples with macrophages. This inspired us that extracting glass fibre from quartz filter membranes directly appears to be an excellent option.
Our team began experiments by observing the frustrated phagocytosis behaviour of the cells. Through bright-field and confocal videos, we observed the whole process of phagocytosis of glass nanofibres by macrophages with an evident extension of cell bodies along the fibre axes to eventually adopt stretched and polarized shapes. Importantly, clearly visible actin rings appeared at the poles of the active polarized macrophages at the very place where they connected to the unengaged parts of the glass nanofibres, suggesting the formation of phagocytic cups (Fig. 1b). Transmission electron microscopy (TEM) confirmed macrophage internalization of glass nanofibres (Fig. 1c). These experiments perfectly revealed that the macrophages could not wholly engulf the glass nanofibres and displayed all characteristics of “frustrated phagocytosis”.
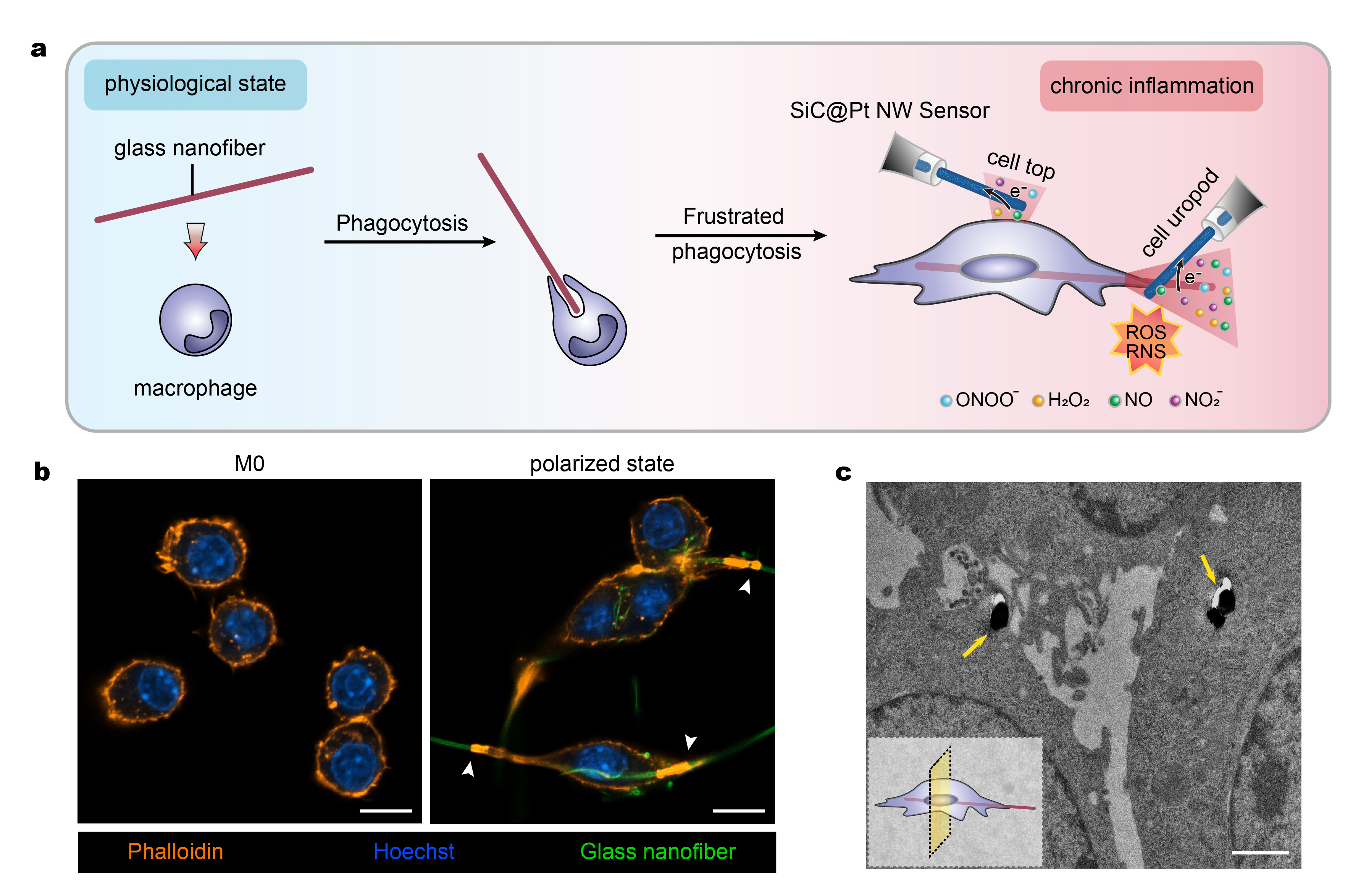 Fig. 1 a) Schematic diagram describing the frustrated phagocytosis of a glass nanofibre by a macrophage and the use of a SiC@Pt NW sensor for differentiating ROS/RNS released. b) Morphology of macrophages before (M0 state) and 12 h after frustrated phagocytosis (polarized state) of glass nanofibres. The highlighted orange areas pointed by the white arrowheads represent the actin rings present at the phagocytic cups. Scale bar, 10 μm. c) TEM of macrophages and after 12 h of frustrated phagocytosis of glass nanofibres (shown by yellow arrows); Scale bar, 1 μm.
Fig. 1 a) Schematic diagram describing the frustrated phagocytosis of a glass nanofibre by a macrophage and the use of a SiC@Pt NW sensor for differentiating ROS/RNS released. b) Morphology of macrophages before (M0 state) and 12 h after frustrated phagocytosis (polarized state) of glass nanofibres. The highlighted orange areas pointed by the white arrowheads represent the actin rings present at the phagocytic cups. Scale bar, 10 μm. c) TEM of macrophages and after 12 h of frustrated phagocytosis of glass nanofibres (shown by yellow arrows); Scale bar, 1 μm.
Enthusiastic by these findings, we positioned the tip of the SiC@Pt NW sensor at specific locations on cell body surfaces and at different stages (0, 4, 12, 20 and 24 h) of their frustrated phagocytosis of glass nanofibres to study the intensities and chemical nature of ROS/RNS fluxes emitted by macrophages (Fig. 1a). The electrochemical investigations established that each primary ROS/RNS release near the phagocytic cups differed significantly throughout frustrated phagocytosis, exhibiting a continuous increase during its early stages until reaching a peak at ca. 20 h (Fig. 2a). Due to the complete envelopment of phagocytic cups at 24 h, the ROS/RNS leakage declines drastically (Fig. 2a). Presumably, such complete encapsulation phases would last for longer durations in the case of longer nanofibres but this was not tested to maintain a statistical unity in our investigations.
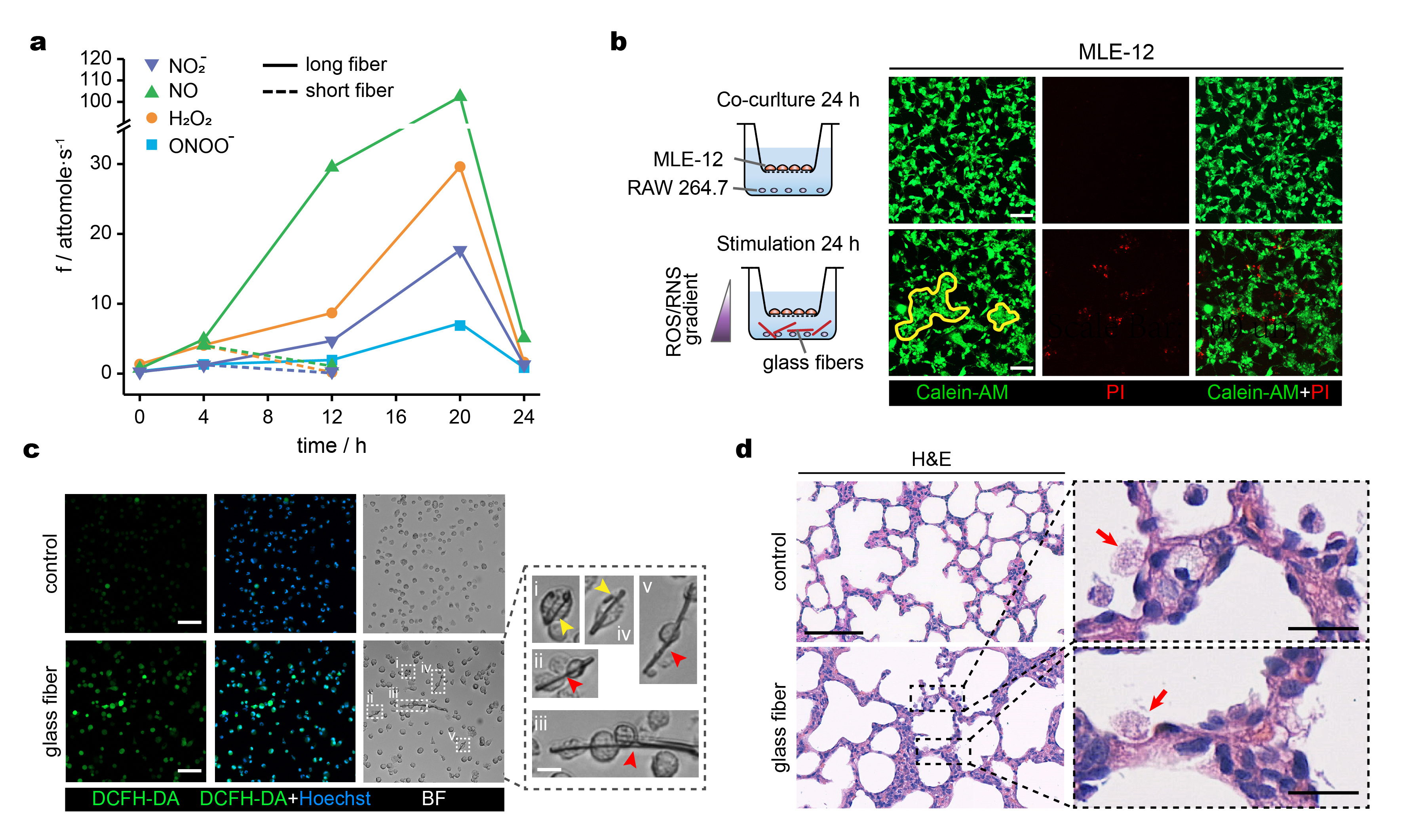 Fig. 2 a) Time variation of the production rates of the four primary ROS/RNS released near a macrophage phagocytic cup during phagocytosis of a long and short glass nanofibre. b) Co-culture assay and calcein-AM and PI fluorescent staining of MLE-12 cells with or without RAW 264.7 cells exposure to glass nanofibres; yellow contours delineate clusters of aggregated cells; scale bar, 100 μm. c) Bright-field and fluorescence microphotographs of alveolar macrophages stained with DC-FHDA (ROS probe) with magnified images of single macrophages undergoing phagocytosis (yellow arrowheads) or co-phagocytosis (red arrowheads) of long glass nanofibres; scale bars, 50 μm and 10 μm. d) Representative H&E staining of lung sections with and without intratracheal glass nanofibres instillation for 14 days; alveolar macrophages pointed by the red arrows in the magnified image; scale bars, 100 μm (left), 20μm (right).
Fig. 2 a) Time variation of the production rates of the four primary ROS/RNS released near a macrophage phagocytic cup during phagocytosis of a long and short glass nanofibre. b) Co-culture assay and calcein-AM and PI fluorescent staining of MLE-12 cells with or without RAW 264.7 cells exposure to glass nanofibres; yellow contours delineate clusters of aggregated cells; scale bar, 100 μm. c) Bright-field and fluorescence microphotographs of alveolar macrophages stained with DC-FHDA (ROS probe) with magnified images of single macrophages undergoing phagocytosis (yellow arrowheads) or co-phagocytosis (red arrowheads) of long glass nanofibres; scale bars, 50 μm and 10 μm. d) Representative H&E staining of lung sections with and without intratracheal glass nanofibres instillation for 14 days; alveolar macrophages pointed by the red arrows in the magnified image; scale bars, 100 μm (left), 20μm (right).
If actual in vivo situations paralleled those monitored in electrochemical discovery, the surrounding tissues on which the nanofibres settled would have been subjected to prolonged destructive fluxes of ROS/RNS, thus suffering severe damages. Based on this, we carried out co-culture and in vivo experiments for validation. Consistent with our hypothesis, intense and prolonged ROS/RNS fluxes released by macrophages led to severe damage to co-cultured lung epithelial cells (Fig. 2b). Additionally, the high fluorescence intensities of ROS and NO confirmed the corresponding intense ROS/RNS spillage in alveolar macrophages collected from the Sprague-Dawley rats (Fig. 2c). Moreover, H&E staining confirmed the chronic inflammation in the lung section (Fig. 2d).
Therefore, the highly sensitive electrochemical nano-sensing approach reported in our work quantitatively and kinetically proved and supported previous hypotheses that lung injury associated with inhalation of chemically inert nanofibres occurs due to the action of prolonged massive fluxes of ROS/RNS emitted by macrophages undergoing frustrated phagocytosis of high aspect ratio inert nanomaterials.
References
- Poland, C. A. et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 3, 423-428 (2008).
- Saito, N. et al. Safe clinical use of carbon nanotubes as innovative biomaterials. Chem. Rev. 114, 6040-6079 (2014).
- Qi, Y. T. et al. Homeostasis inside single activated phagolysosomes: Quantitative and selective measurements of submillisecond dynamics of reactive oxygen and nitrogen species production with a nanoelectrochemical sensor. J. Am. Chem. Soc. 144, 9723-9733 (2022).
Follow the Topic
-
Nature Nanotechnology

An interdisciplinary journal that publishes papers of the highest quality and significance in all areas of nanoscience and nanotechnology.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in