Nanostructured polymer films with metal-like thermal conductivity
Published in Chemistry

Polymers continue to infiltrate into modern technologies ranging from microelectronics to dreamliner airplane, thanks to their unique combination of properties not available from other materials. They are lightweight and easy to process. Traditional polymers are also thermal insulators, a characteristic greatly desired for Styrofoam coffee cup. But it is quite a nuisance for microelectronic industry, etc., because undissipated heat causes undesirable overheating and harms the device performance.
Conventional wisdom of improving polymer’s heat conducting performance is to add thermally conductive fillers (metal nanoparticles, etc) in polymers. But this thermal conductivity enhancement is very limited. The enhancement is just one order of magnitude due to the high interface thermal resistance between fillers and polymers, and thermal insulative property in polymer matrix. Past simulations have predicted that oriented pure polymer could have a very high, even a divergent thermal conductivity. Nine years ago, we successfully demonstrated that ultra-drawn polyethylene nanofibers (~100 nm) can transfer heat more efficiently than many metals. But translating this remarkably high thermal conductivity seen in simulation as well as in these ultra-drawn polyethylene nanofibers into a scalable polymer for application purposes is challenging. First, thermal transport mechanisms in polymers have been elusive. Second, ubiquitous defects such as chain entanglements (Fig. 1a) act as scattering heat-carrier transport in polymers. Overcoming these challenges will expand the scope of polymer use in thermal management and energy systems, since practical applications such as heat exchangers require large areas or volumes of materials.
In this research, inspired by the long-range ordered carbon-carbon covalent bonds in dimond, one of the most thermally conductive materials, we engineered polyethylene films with an emphasis on minimally entangling the chains by Couette-flow extrusion and maximally aligning them by mechanical stretching (Fig. 1b).
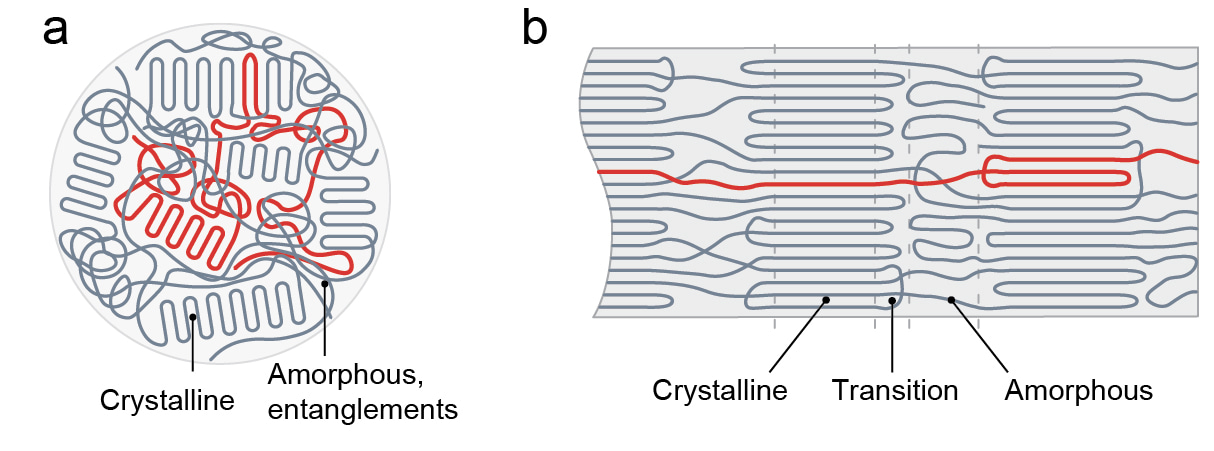
Figure 1.(a) Thermally insulative bulk polymers have disordered and entangled chains. (b) Thermally conductive polymers have ordered and disentangled chains.
We achieved a metal-like thermal conductivity of ~62 W/m-K (Fig. 2a), which is two orders of magnitude greater than conventional polymers (0.1-0.5 W/m-K). These films are more thermally conductive than many metals (304 stainless steel ~15 W/m-K) and ceramics (aluminum oxide ~30 W/m-K). We also discovered the underlying thermal transport mechanism. With the help of intense and high-resoluiton synchrotron X-ray scattering, we were able to quantify the structures of the crystalline and amorphous phases in the nanofibers that make up the film. With a linear thermal model, we uncovered the secret of such a metal-like polymer performance—the amorphous region of the nanofibers (Fig. 1b) has a surprisingly high thermal conductivity, over 16 W/m-K (Fig. 2b), which is central to the overall high thermal conductivity. While previous findings revealed that the crystalline region is important for the thermal conductivity, we foresee that further improvement of the thermal conductivity of the amorphous phase will be the key to developing the next generation of heat-conducting polymers. Such metal-like heat conducting polymers with their other unique combination of characteristics (light weight, optical transparency, chemical stability etc.) hold promise for many existing and potential applications, for example, microelectronics cooling and thermal energy storage systems.
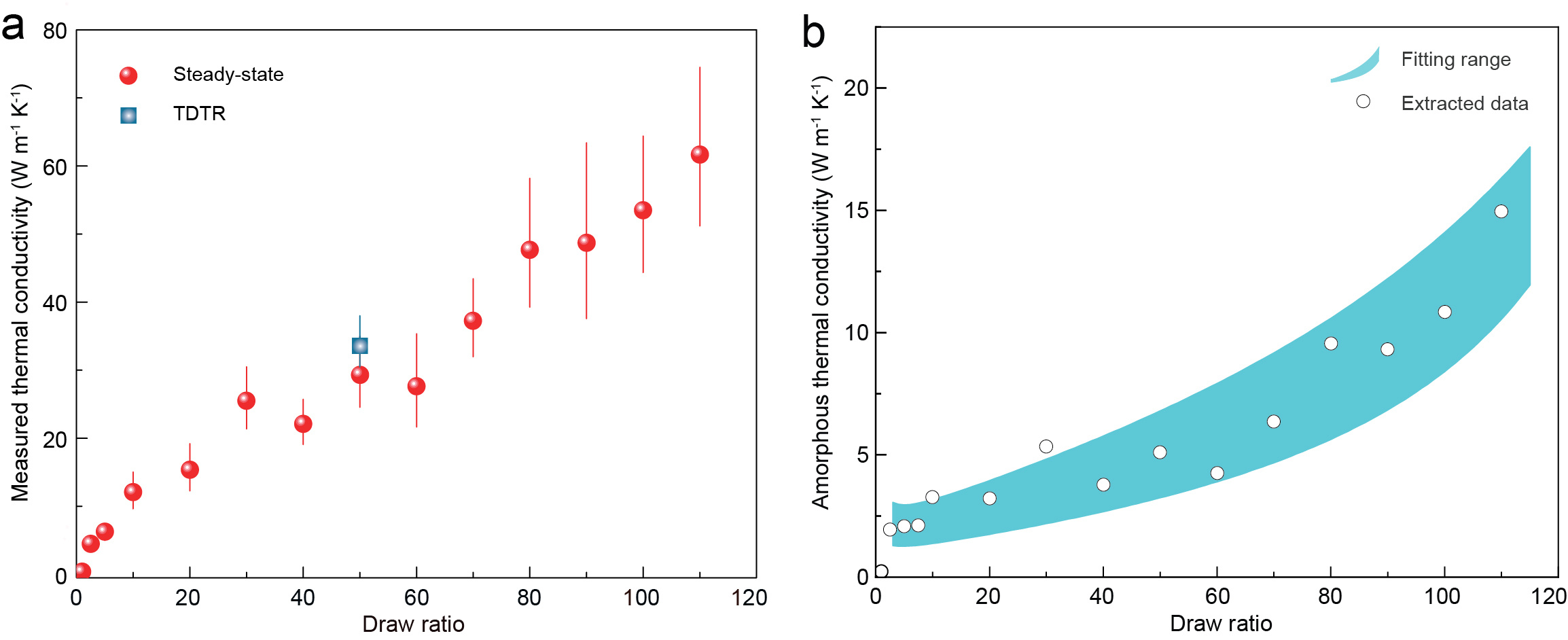
Figure 2.(a) Measured and (b) computed thermal conductivities for the polymer films.
For more details, please check out our paper “Nanostructured polymer films with metal-like thermal conductivity” on Nature Communications. https://www.nature.com/articles/s41467-019-09697-7
This blog is contributed by Yanfei Xu, Jiawei Zhou, Daniel Kraemer, Zhang Jiang and Gang Chen* (gchen2@mit.edu).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in