Nature keeps inspiring us
Published in Chemistry

Today the great challenge is to promptly cure organ failures and offer patients a better outcome after-surgery process, which is often complicated by tissue distortions, bleeding and high infection rates due to the type of sutures, wires and staples used in standard medical procedure.
In order to overcome these drawbacks, tissue engineering1,2 has proposed a brand-new approach which is able to revolutionize the entire medicine: the development of bioadhesive on which cells can attach and proliferate, reconstructing a damaged tissue or an entire organ but also to simplify the procedure, time of recuperation as well as lowering mortality rates3. The future bioadhesives have to satisfy specifically criteria, including the ability to interact with surfaces with different make-up, maintain adhesive properties, possess antimicrobial behavior, resist to mechanical strains, be biocompatible and, finally but most important, work in wet environment.
In this contest, scientists have taken inspiration by Nature, which has always been a main source of inspiration for human-being. Nature is full of animals for which adhesion is crucial for their life as a part of their defensive strategy or for attachment on different surfaces. Among all organisms able to secrete adhesive products, mussels are able to firmly adhere to wet surface thanks to the byssus plaque, a protein-base appendage, which can be considered as a versatile underwater adhesive between the mussels byssal threads and the marine surface. The formation of the byssus plaque involves several proteins4,5 (mussel foot proteins) which participate to a complex process, among which the formation of coacervates6,7, small liquid droplets of two immiscible liquid phases, is considered one of the most important steps. In nature, mussel foot proteins, involved in surface contacts, are rich in Dopamine (DOPA) residues8, generated by a post-translational modification of tyrosines. The presence of the catechol moiety is considered fundamental for the coacervation phase and for the interaction with the surface in addition to lysine side chain flanking DOPA, able to attracting water molecules and leaving the surface free for the interaction with DOPA9.
In the last decade, many efforts have been done to create strong materials suitable to act as biomedical adhesives, starting from studying the composition of mussel adhesive proteins and trying to mimic their adhesive properties, although the lack of a complete understand of the entire biological process has impaired the finding of such material. One of the reason is the absence of a complete structural characterization of Mussel foot proteins as well their folding, which knowledge can help not only into highlighting how these adhesive proteins work and interact with wet surfaces but also can strongly contribute in designing biomaterials behaving the same as the natural source to be employed in different areas of human health.
To this purpose, we structurally characterized the recently identified byssal plaque adhesive protein from the Perna viridis mussel species, Pvfp-5β10,11; the first to be secreted by mussels and to initiate an interaction with sea surface. The recombinant Pvfp-5β was produced to jump the difficulty in recovering Pvfps proteins from their native source, and, more important, to deal with a no Dopa form of Pvfp-5β and explore adhesive features other than DOPA. Using a biophysical approach, based mainly on Nuclear Magnetic Resonance (NMR) and supported also by an extensive probing of its cell adhesion properties, we demonstrated that it is possible to produce recombinant Pvfp-5β in bacteria and perform a complete structural characterization of Pvfp-5β. We demonstrated it is able to coacervate and evolve in fibrils in a process similar to the formation of the amyloid fiber (Fig.1).
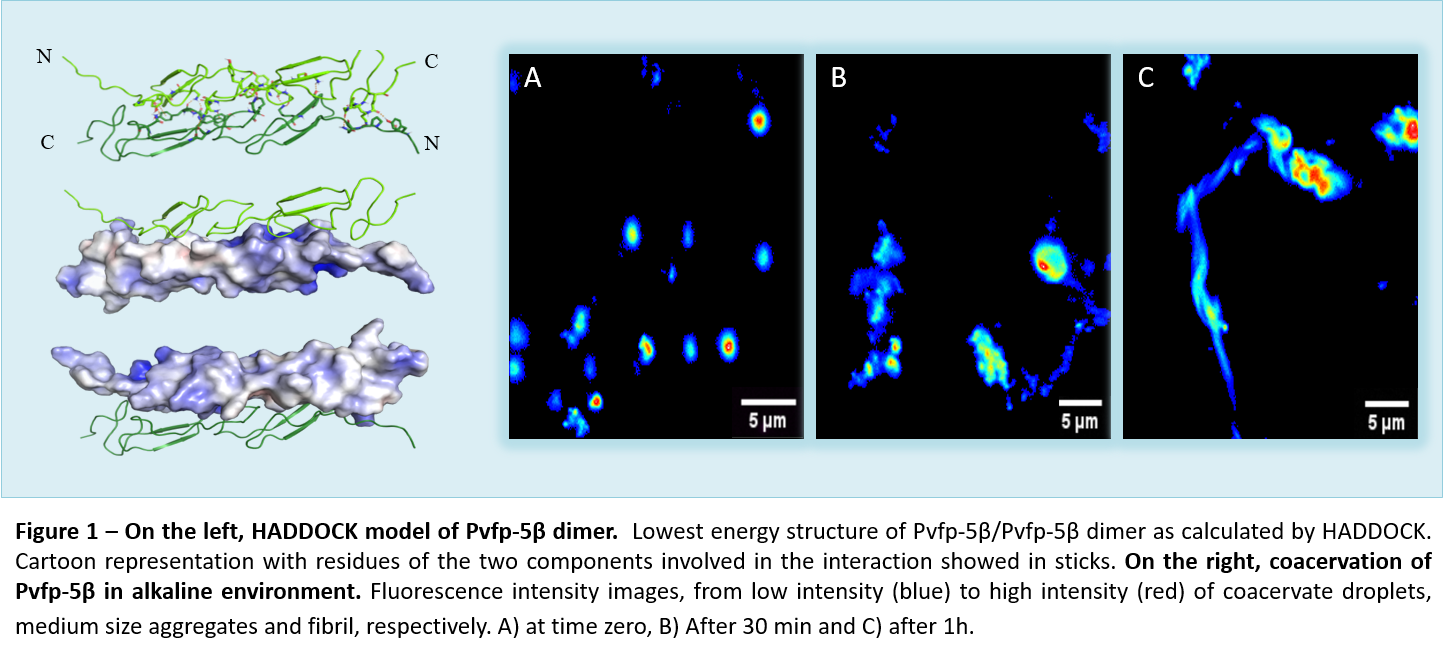
We were able to confirm its low toxicity and intrinsic adhesive properties for mammalian cells without DOPA modifications11. Our evidence on the Pvfp-5β folding and residues distribution can help into explain why it is the frontline protein in the process of adhesion in Perna viridis. Its elongated shape and the exposure of so many interaction hotspots are ideal for adhesion to marine substrates. Also, our computational calculations furnish a 3D detailed picture of the possible interactions in coacervate formation (Fig.1). Moreover, our results help into reduce the role of DOPA into coacervation and adhesion process and into stand out the importance of a secondary structural features and the presence of hydrophobic residues. In conclusion, our work constitutes a direct structural attempt to understand the molecular recognition of mussel proteins at the molecular level and provides a model for mussel byssal plaque formation, that could be exploit for the development of a future potential bio-adhesive.
References
- Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3(10):589-601. doi:10.1098/rsif.2006.0124
- Rusk RD. Science. Educ Forum. 1950;15(1):119-120. doi:10.1080/00131725009342110
- Pandey et al. Mussel-inspired bioadhesives in healthcare: design parameters, current trends, and future perspectives. Biomater. Sci. 2020; 8(5): 1240-1255. doi: 10.1039/C9BM01848D
- Waite JH. Mussel adhesion - Essential footwork. J Exp Biol. 2017;220(4):517-530. doi:10.1242/jeb.134056
- Priemel T, Degtyar E, Dean MN, Harrington MJ. Rapid self-assembly of complex biomolecular architectures during mussel byssus biofabrication. Nat Commun. 2017;8(March):1-12. doi:10.1038/ncomms14539
- Astoricchio E, Alfano C, Rajendran L, Temussi PA, Pastore A. The Wide World of Coacervates: From the Sea to Neurodegeneration. Trends Biochem Sci. 2020;45(8):706-717. doi:10.1016/j.tibs.2020.04.006
- Abbas, M., Lipin´ski, W. P., Lipin´ski, L., Wang, J. & Spruijt, E. Peptide-based coacervates as biomimetic protocells. Chem. Soc. Rev. 2021; 50: 3690-3705. doi: 10.1039/D0CS00307G
- Yu M, Hwang J, Deming TJ. Role of 1-3,4-dihydroxyphenylalanine in mussel adhesive proteins. J Am Chem Soc. 1999;121(24):5825-5826. doi:10.1021/ja990469y
- Shin M, Shin JY, Kim K, et al. The position of lysine controls the catechol-mediated surface adhesion and cohesion in underwater mussel adhesion. J Colloid Interface Sci. 2020;563:168-176. doi:10.1016/j.jcis.2019.12.082
- Petrone L, Kumar A, Sutanto CN, et al. Mussel adhesion is dictated by time-regulated secretion and molecular conformation of mussel adhesive proteins. Nat Commun. 2015;6:1-12. doi:10.1038/ncomms9737
- Santonocito R, Venturella F, Piaz FD, et al. Recombinant mussel protein Pvfp-5β: A potential tissue bioadhesive. J Biol Chem. 2019;294(34):12826-12835. doi:10.1074/jbc.RA119.009531
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Related Collections
With Collections, you can get published faster and increase your visibility.
From RNA Detection to Molecular Mechanisms
Publishing Model: Open Access
Deadline: May 05, 2026
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: May 31, 2026
Latest Content
Why is Singapore Identified in Global Research as Number One? How Physical Activity and Education Excellence Created a Global Leader






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in