No More Blind Spots: Mapping the Entire Volume of the Human Heart
Published in Bioengineering & Biotechnology and Biomedical Research

The "Flashlight" Problem
Imagine you are in a pitch-black room. You are trying to find a specific object, but you only have a small flashlight. You can see whatever you point at with crystal clarity, but the rest of the room remains a mystery. You have to piece together the layout of the room in your mind, scan by scan, hoping you don't miss anything important in the shadows.
For decades, this has been the reality for electrophysiologists treating cardiac arrhythmias.
Using catheters, doctors enter the heart and map electrical signals point-by-point. They are incredibly skilled at building these maps, but the process can be slow, and fundamentally limited: they can only see where the catheter touches. If the source of the arrhythmia—the "short circuit" causing the heart to beat irregularly—is hiding somewhere the catheter isn't touching, or worse, deep inside the heart muscle wall where a catheter can't reach, they are effectively operating in the dark.
This limitation gnawed at us. We asked ourselves a simple but ambitious question: What if we could turn on the lights in the entire room at once?
The Challenge: Seeing Through Walls
Non-invasive mapping (Electrocardiographic Imaging or ECGI) isn't new. For years, scientists have used body surface electrodes to reconstruct heart activity without surgery. However, traditional ECGI had a major flaw: it treated the heart like a hollow shell, only estimating signals on the outer surface.
But the heart is a thick, three-dimensional muscle. The most dangerous arrhythmias, such as Ventricular Tachycardia (VT)—a leading cause of sudden cardiac death—often originate deep within the walls (the mid-myocardium) or in the septum separating the chambers. Existing models were like looking at a building from the outside and trying to guess what was happening in a room on the third floor. We needed a way to see through the walls.
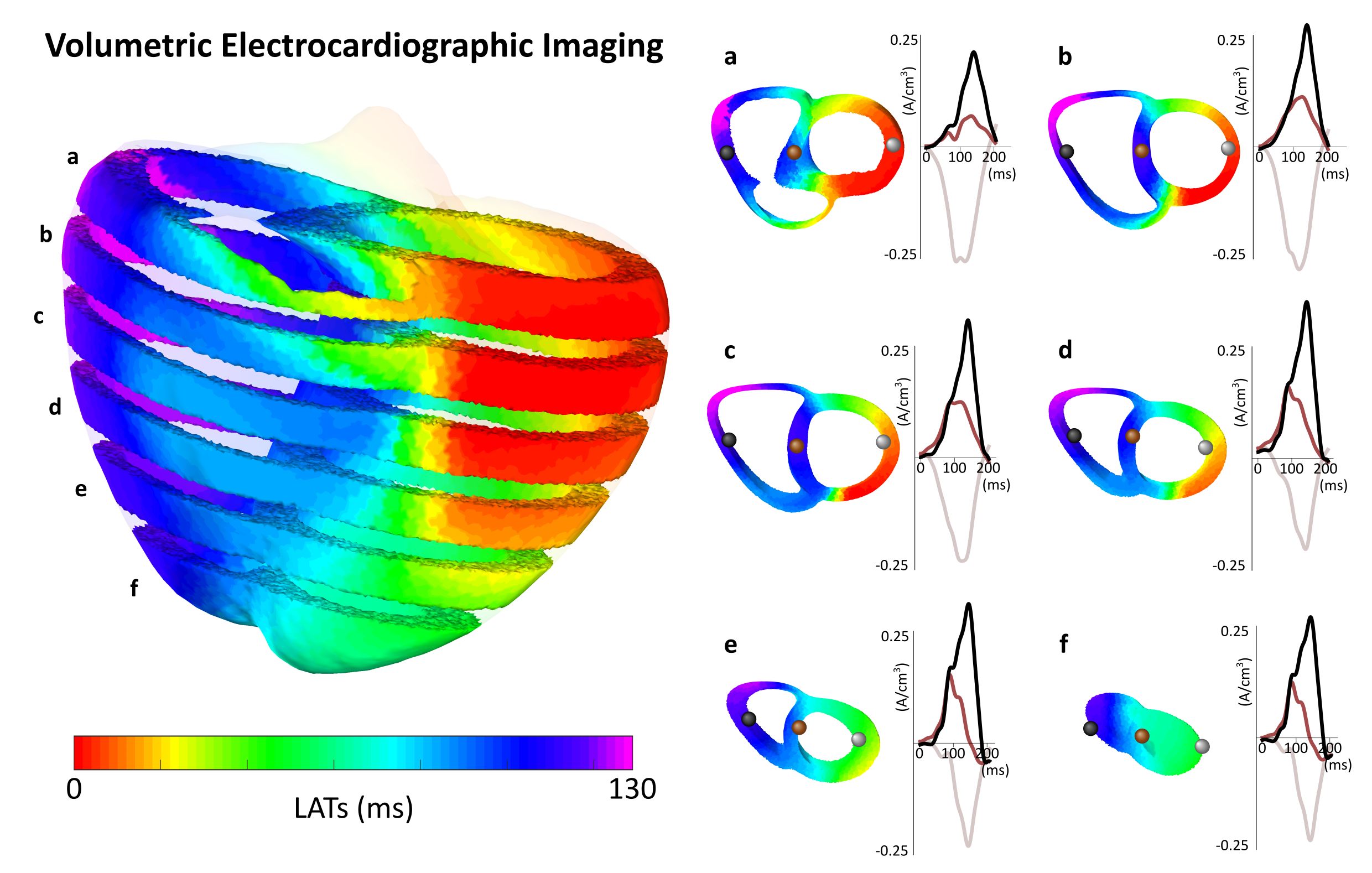
Figure 1: Seeing Through the Walls. Unlike traditional models that treat the heart as a hollow shell, VSI allows us to visualize the "engine inside." These cross-sections demonstrate our ability to decouple electrical signals originating from the inner layers (deep myocardium) from those on the surface. This volumetric depth allows us to reconstruct activity throughout the entire thickness of the muscle wall.
The Breakthrough: Volumetric Source Imaging
Our journey to this Nature Communications Medicine paper began with a shift in perspective. Instead of calculating electrical potentials on the surface, we developed a novel Volumetric Source Imaging (VSI) technology to reconstruct activity throughout the entire volume of the heart.
Think of it as a "Digital Twin" approach. We take electrical signals from the patient's torso and the geometry of their heart and use mathematical algorithms to solve the physics in reverse. The breakthrough was proving that we could decouple signals coming from the inner layers of the heart from those on the outer layers. Suddenly, we weren't just seeing the shell; we were seeing the engine inside.
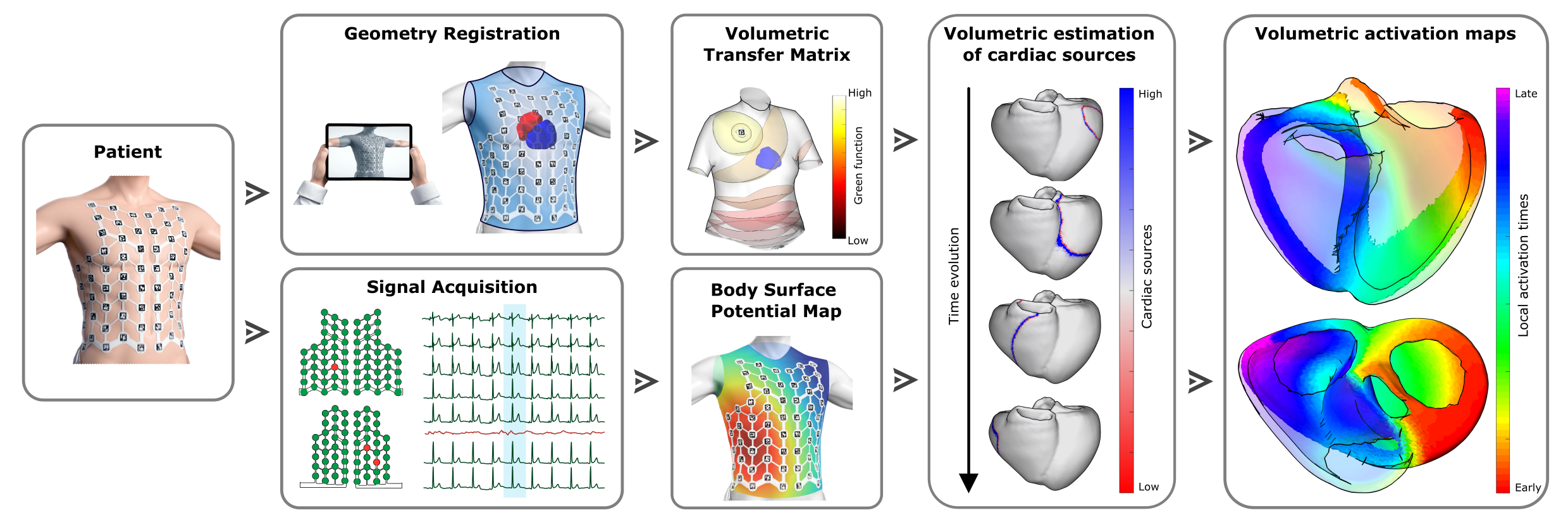
Figure 2: The Estimation Workflow. This framework illustrates our novel Volumetric Source Imaging (VSI) pipeline, developed in collaboration with the startup Corify Care S.L. By combining non-invasive signal acquisition from the torso with the patient's specific heart geometry, we apply mathematical algorithms to solve the physics in reverse. This process transforms surface data into a complete 3D volumetric activation map, effectively allowing us to "turn on the lights" in the entire heart without a single incision.
From Theory to Clinical Clarity
Validation was the true test. We started with computer simulations, but the real challenge was the hospital. Working with clinical teams at Hospital Gregorio Marañón and Hospital Clínic de Barcelona, we tested the system on real patients.
I remember one specific patient with sustained Ventricular Tachycardia (VT). VT is critical because it is often driven by scars deep in the heart muscle. If doctors can't find and ablate these circuits, the patient remains at high risk of sudden death. In this specific case, the arrhythmia was hiding in the septal wall—a notorious "blind spot" for traditional non-invasive tools.
When we applied our system, the screen lit up. In a single heartbeat, the 3D map clearly visualized the re-entrant circuit spinning inside the septal wall. It matched the invasive data perfectly.
That was the moment we knew: this isn't just math; this is Clinical Clarity.
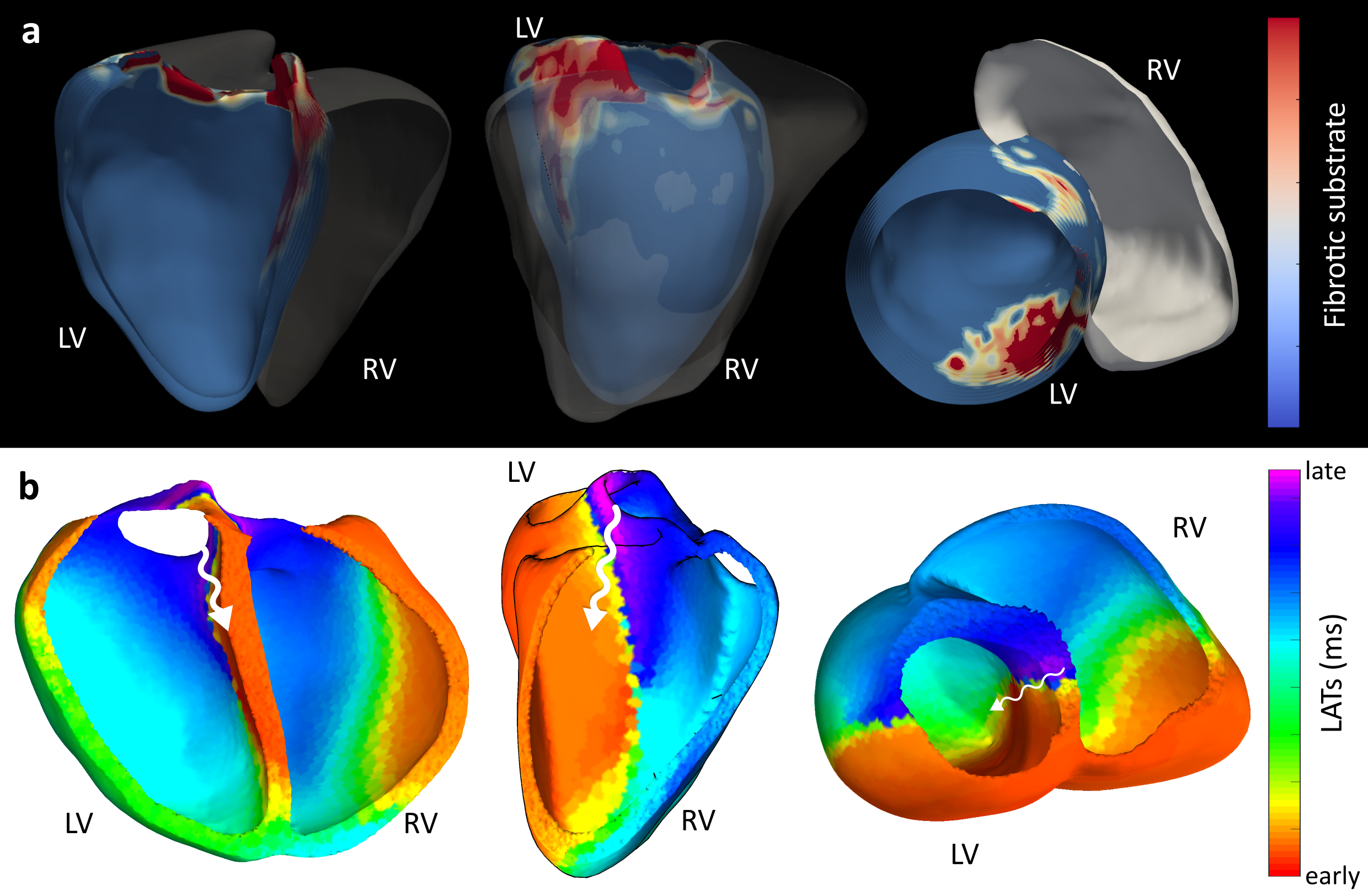
Figure 3: Clinical Clarity in Action. A real-world validation of a complex arrhythmia located at the ventricular septum- a notorious “blind spot” for standard tools. The image on the top (a) shows the fibrosis map created with a tool called ADAS, which highlights the "scars" in the heart tissue where arrhythmia often start. The image at the bottom (b) shows our non-invasive VSI map. In a single heartbeat, our system pinpointed the re-entry, matching the location of the scar with high precision.
The Future of the EP Lab
The implications of this research go far beyond a nice 3D image:
- Speed and Safety: Mapping the whole heart in a single beat non-invasively can reduce procedure time and X-ray exposure.
- No More Blind Spots: We can finally visualize the septum and mid-myocardium.
- Democratization: Non-invasive software can make high-precision diagnostics accessible to more hospitals, helping more patients get the right treatment the first time.
This publication is not the finish line; it is the validation we needed to scale. We are moving toward a future where the diagnostic phase happens before the patient enters the operating room. We are turning the lights on. And once you see the whole picture, you can never go back to the flashlight.
Follow the Topic
-
Communications Medicine

A selective open access journal from Nature Portfolio publishing high-quality research, reviews and commentary across all clinical, translational, and public health research fields.
Related Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Healthy Aging
Publishing Model: Open Access
Deadline: Jun 01, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in