Not all bacterial LPS are bad: a look at the structure and role of Akkermansia muciniphila's unique LOS
Published in Microbiology, Sustainability, and Biomedical Research
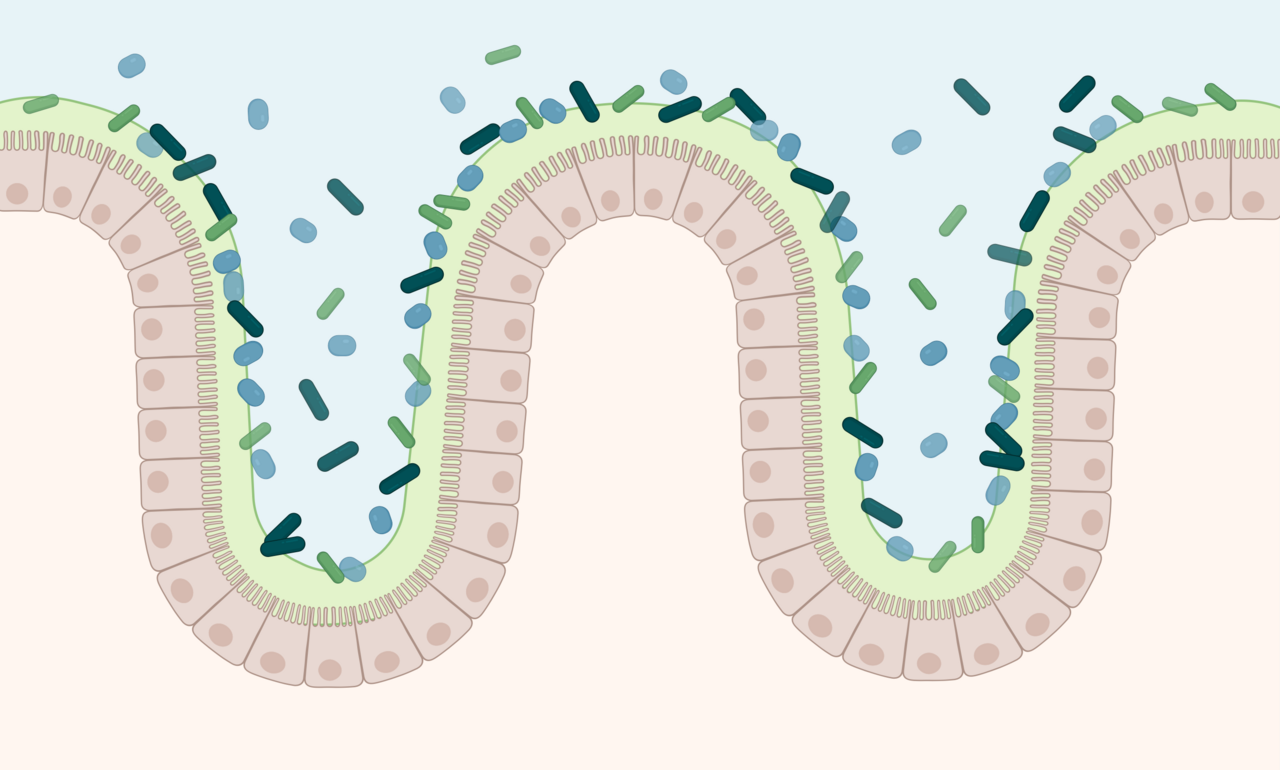
In the world of bacteria, certain molecules tend to stand out for their ability to impact our immune system—these include Lipopolysaccharides (LPS). Traditionally, LPS has had a bad reputation. This molecule, present in the cell walls of Gram-negative bacteria, can provoke intense immune responses when it crosses into our bloodstream, sometimes leading to serious conditions like sepsis. For a long time, Gram-negative bacteria were therefore considered ‘bad’ bacteria. Yet, some, as we show here, can actually play a positive role in host’ health, particularly in the gut.
One example is Akkermansia muciniphila, a Gram-negative bacterium that resides in the gut and is known to offer numerous health benefits. As part of an international and multidisciplinary team of researchers from Naples, Italy (led by Tony Molinaro), Brussels, Belgium (led by Patrice D. Cani), and Wageningen, The Netherlands (led by Willem M. de Vos), we have found that Akkermansia muciniphila MucT produces Lipooligosaccharides (LOS) with unique chemical structure and signaling capacity that show an anti-inflammatory activity.
Our recent studies are shedding light on how this microbe, and particularly its LPS-like structure containing a unique set and configuration of sugars, may promote gut health and help balance immune responses. This surprising discovery flips the script on LPS and opens up new possibilities for therapies related to gut health and inflammation.
What are LPS and LOS and why are they often seen as harmful?
LPS, also known as endotoxin, is found in the outer membrane of Gram-negative bacteria. It serves as a structural component but also acts as a powerful immune system trigger. Some bacteria produce a special form of LPS called lipooligosaccharide (LOS), which is structurally similar but lacks the long O-antigen polysaccharide chain. This modification results in a shorter structure that still retains the ability to activate the host immune system.
When LPS or LOS enters the bloodstream, it can bind to specific immune receptors called Toll-Like Receptors (TLRs), particularly TLR4, causing an immune response. This response is usually inflammatory and this can lead to chronic inflammation, metabolic disorders, or severe infections.
In healthy individuals, our intestinal barrier prevents large amounts of harmful LPS/LOS from entering the bloodstream. But in some conditions, like obesity or type 2 diabetes, this barrier weakens —often refered to as a "leaky gut"—, allowing high amounts of LPS/LOS to enter the bloodstream and circulate, contributing to inflammation in many tissues and to various heath complications and metabolic issues. It is no wonder LPS/LOS are often considered the villains in discussions about gut health and immune response.
Enter Akkermansia muciniphila: a symbiont with a special LOS
While most Gram-negative bacteria produce harmful LPS/LOS, A. muciniphila turns out to be an exception. This bacterium lives in the mucus layer of the intestines and is essential for maintaining a healthy gut barrier. Studies in both mice and humans have shown that having plenty of A. muciniphila in the gut is linked to better barrier function, reduced inflammation, and even protection against obesity and metabolic diseases.
So, how does A. muciniphila, a Gram-negative bacterium, manage to be beneficial? While several mechanisms have been proposed to explain how A. muciniphila benefits its host’s health, one key factor may be its distinctive form of LOS. Unlike the LOS of other bacteria, A. muciniphila's LOS contains a unique combination of sugars, including fucose—a sugar rarely found in bacterial LOS—which may contribute to its unique effects.
The unique structure of Akkermansia muciniphila's LOS
Our research has revealed that A. muciniphila’s LOS is composed of a highly intricate carbohydrate structure that does not follow the typical patterns seen in other bacteria. It contains two complex glycan chains and a mixture of differently modified lipid molecules that contribute to its function. Instead of provoking a strong inflammatory response, this LOS interacts with the immune system in a much gentler way.
What makes A. muciniphila even more fascinating is how its LOS signals through not just the usual TLR4 receptor, but also through TLR2. Typically, TLR4 is the receptor responsible for recognizing harmful LPS/LOS, while TLR2 is associated with recognizing more beneficial signals. By interacting with both, A. muciniphila’s LOS helps create a balanced immune response that might prevent it from tipping into harmful inflammation.
In experiments with mice, when we injected A. muciniphila’s LOS, we observed a significant increase in the expression of anti-inflammatory genes, particularly IL-10, a cytokine known for calming inflammation. Even more impressive, this LOS activated TLR2 a hundred times more than it activated TLR4. This unique signaling ability is thought to be one of the reasons why A. muciniphila helps maintain a healthy gut environment while avoiding the negative effects typical of other Gram-negative bacteria.
Why does this matter for human health?
The ability of A. muciniphila to promote gut health and reduce inflammation is increasingly important as researchers and clinicians explore new ways to treat conditions like obesity, diabetes, and chronic gut inflammation. One of the main problems with these conditions is a disrupted gut barrier, which allows harmful LPS/LOS from other bacteria to leak into the bloodstream and trigger inflammation.
By strengthening the gut barrier and promoting anti-inflammatory immune responses, A. muciniphila may help prevent this "leaky gut" scenario. Even more exciting is the fact that pasteurized (heat-killed) A. muciniphila cells seem to retain these benefits, possibly due to the stability of its LOS and other outer membrane proteins.
In human studies, patients with metabolic disorders who received A. muciniphila (either live or pasteurized) showed improvements in gut barrier function and metabolic markers, along with lower levels of harmful circulating LPS. This suggests that A. muciniphila could potentially be used as a probiotic or even as a treatment to help people with these conditions.
Rethinking the role of LPS: from foe to friend
The discovery of A. muciniphila’s unique LOS highlights the complexity of our relationship with gut bacteria and challenges the idea that all LPS/LOS are harmful. Instead, we’re learning that some forms, like the one produced by A. muciniphila, can actually help regulate immune responses and promote health.
This research is still in its early stages, but it opens up exciting possibilities for the future. Could we one day see therapies designed to mimic or enhance the beneficial effects of A. muciniphila’s unique LOS? What about the LPS/LOS of other bacteria? How do their unique structures impact our gut barrier and immune system?
As we continue to unravel the intricacies of gut bacteria and their impact on human health, it seems clear that not all LPS/LOS are created equal and may play a central role in this evolving story that reflects the evolutionary adaptation of A.muciniphila as mucolytic symbiont.
So, next time you hear about LPS, remember: it’s not always the bad guy. In the case of Akkermansia muciniphila, it might just be one of the keys to a healthier gut!
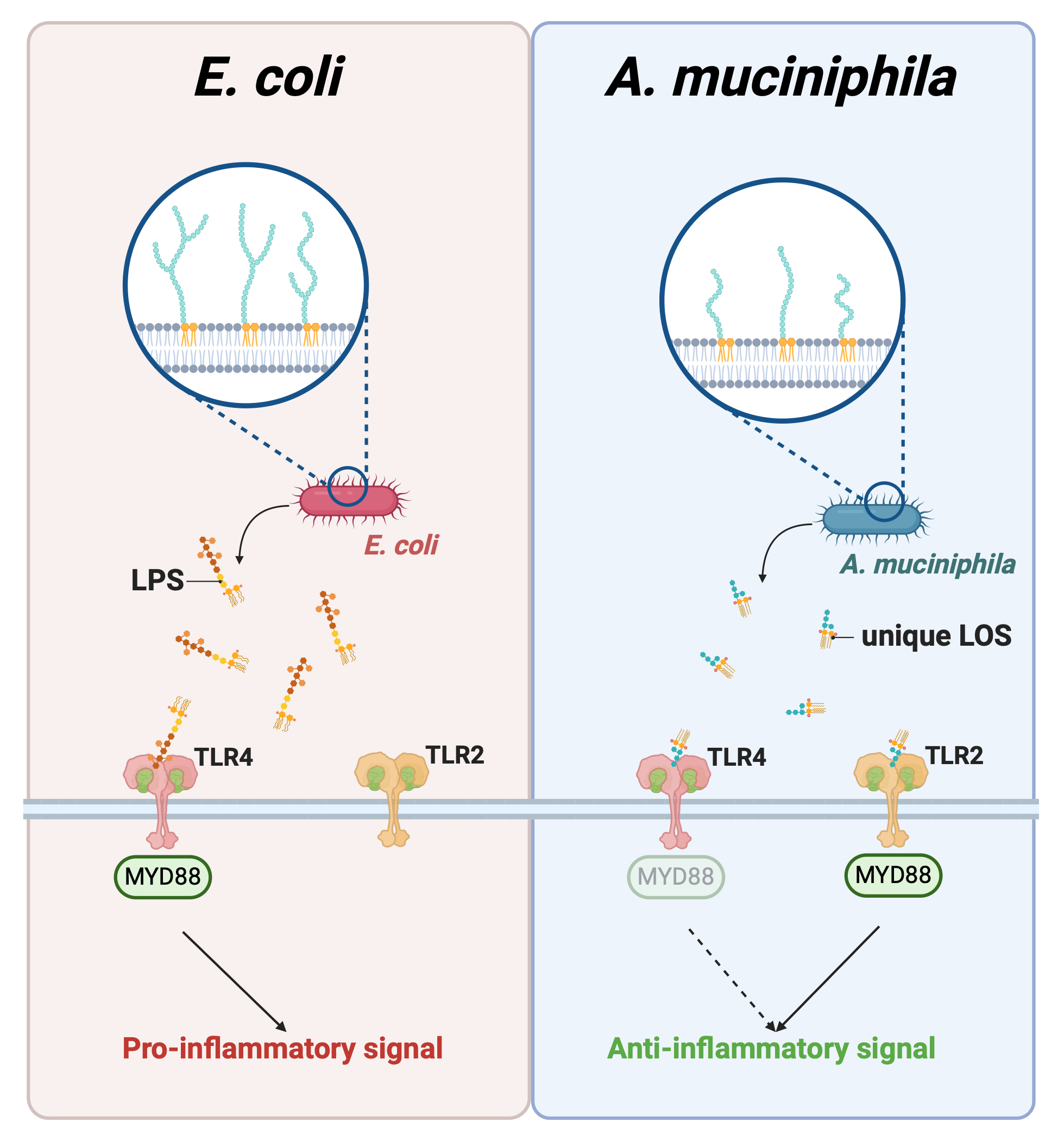
Proposed mechanism of Akkermansia muciniphila's unique LOS compared to pathogenic E. Coli's LPS. (Created in BioRender.com)
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in