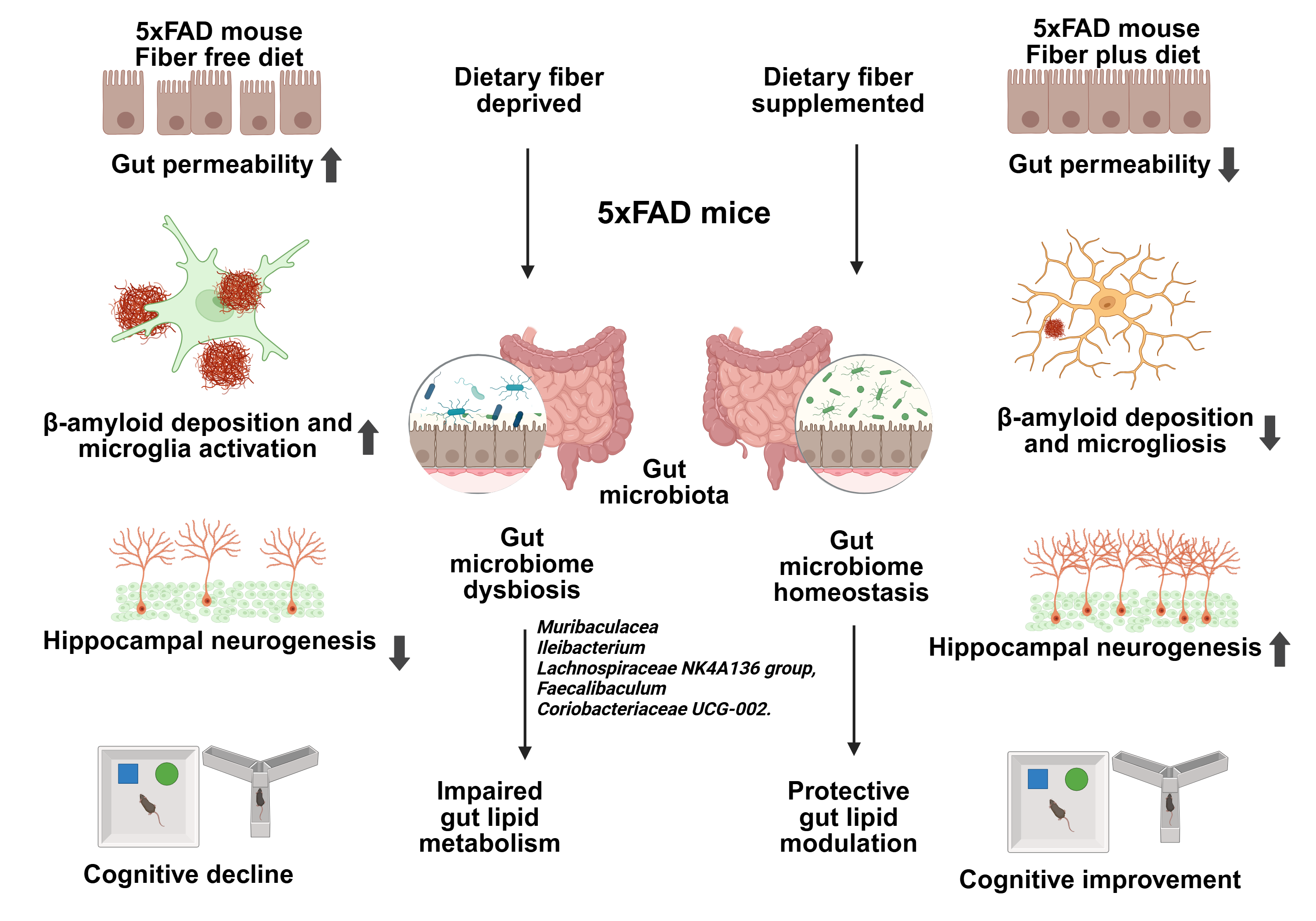
Our paper, Zhao et al. [1], reports that a diet supplemented with certain fibers regulates gut microbiota and lipid metabolism and, as a consequence, counteracts Alzheimer’s pathology and cognitive deficits in 5xFAD mouse that carries mutations in five genes of familial AD and develops AD-like pathology, including abundant amyloid plaques in the cortex and hippocampus, enhanced microgliosis, neuroinflammation, neuronal loss, and cognitive impairments. This discovery offers the application of nutrition, because diets selectively remodel the gut, promoting a microbial environment that favors metabolic and anti-inflammatory consequences.
In our laboratory, we strive to understand how the body’s organs function not in isolation, but as a highly coordinated, interconnected axis system. As life expectancy continues to rise, unraveling this systems-level approach becomes increasingly vital for preserving quality of life amid chronic and complex diseases.
One compelling example is the brain–heart axis, which bridges neuroscience and cardiology. It helps explain Takotsubo cardiomyopathy, or “broken heart syndrome,” where acute emotional stress leads to cardiac dysfunctions. This connection underscores the importance of cross-disciplinary research in decoding human health.
Another frontier being explored is the brain’s role in immune surveillance. The brain doesn’t just passively receive signals—it actively coordinates immune responses. When functioning properly, this protects the body; when misregulated, it can contribute to disease. This insight opens therapeutic avenues for conditions like Long Covid and other immune-related disorders, allowing the decoding of the body’s complex networks and the development of interventions that extend not only lifespan but also health-span.
One of these exciting areas of discovery lies in the power of nutrition to shape brain health. In our study [1], we show that certain dietary fibers modulate the gut microbiota and lipid metabolism, thus offering a non-invasive strategy to prevent or slow the progression of neurodegeneration. Using an Alzheimer’s disease (AD) model, our findings suggest that fiber’s impact on the gut–brain axis could help reduce the onset and progression of Alzheimer’s and likely other neurodegenerative diseases, including Parkinson’s.
This research represents a transformative step forward in understanding how diet, microbiota, and lipid signaling converge to influence brain health. It’s a testament to the power of systems biology—and a hopeful glimpse into the future of neurodegenerative disease prevention.
The gastrointestinal (GI) tract harbors a vast microbial ecosystem—its bacterial population exceeds the total number of human cells in the body. While some microbes are beneficial, promoting immune and neurological health, others are detrimental. In our study, we used 16S rRNA sequencing to analyze fecal samples from mice fed either fiber-rich or fiber-free diets [2–4]. This approach revealed a shift in microbial composition, with fiber supplementation promoting protective populations, such as Muribaculaceae and Ileibacterium, which are associated with improved gut and brain health.
“Adoptive transfer” of obese gut microbiota from obese mice into lean mice induced behavioral changes in lean mice (in the absence of induced obesity) [3]. This gut microbial transfer increased anxiety in the mice and promoted compulsive behaviors. Also, Limosilactobacillus reuteri ameliorates maternal separation stress in newborn mice and alters subsequent adult behavior [4].
At the phylum level, we observed a reduction of Actinobacteriota after fiber supplementation, suggesting that fiber intake reshapes microbial communities even at higher taxonomic levels. More differential changes were revealed at the genus level by our diet. For example, Muribaculaceae and Ileibacterium were increased under FP diets. Notably, similar features of a “youth-like” gut microbiome have also been observed in the oldest centenarian that reached 117 years of age, where enrichment of Bifidobacterium and reduced pro-inflammatory signatures were linked to healthy aging.
Faecalibaculum was reduced following FP supplementation is associated with depression, raising the possibility that its suppression reflects a shift toward a healthier microbial community. Moreover, Rikenella and Dubosiella, which were enriched in AD mice, decreased under FP diets. Both taxa have been implicated in pro-inflammatory responses and AD-associated microbiota dysbiosis, suggesting that their reduction may represent a microbiota-mediated mechanism contributing to the attenuation of neuroinflammation. Rikenella has been suggested to be a biomarker for AD. Furthermore, Bacteroides showed divergent responses: enriched in FP-fed WT mice but reduced in FP-fed AD mice. This context-dependent behavior is consistent with the dual role of Bacteroides, which serve as beneficial commensals in healthy hosts but may exacerbate pathology under AD conditions.
Additional fiber-responsive taxa, including Lachnospiraceae and Coriobacteriaceae UCG-002, also exhibited consistent modulation in both WT and AD. Lachnospiraceae is a fiber-responsive beneficial genus and biomarker for better prognosis in stroke patients, while Coriobacteriaceae_2 was implicated in intestinal barrier disruption and pro-inflammatory responses.
The gut microbiota produces and modulates lipid metabolites that can cross the blood–brain barrier and affect brain function. Dietary fiber alters the gut microbiome, which in turn reshapes lipid metabolism in ways that support neuroprotection. Certain lipids, such as omega-3 fatty acids, have anti-inflammatory properties. Our study found increased levels of these lipids in fiber-fed mice, which correlated with reduced neuroinflammation—a hallmark of Alzheimer’s disease.
Lipid metabolism plays a crucial role in brain health and is increasingly recognized as a key factor in the development and progression of Alzheimer’s disease [5,6]. Lipids are essential components of neuronal cell membranes, influencing membrane fluidity, receptor function, and signal transduction [7–9]. Disruptions in lipid metabolism impair these processes, contributing to neurodegeneration [10].
Lipid composition also affects the processing of amyloid precursor protein (APP), which can influence the production of amyloid-beta (Aβ) peptides [11,12]. Altered lipid metabolism may promote Aβ aggregation, while balanced lipid profiles can help reduce deposition. Lipids are vital for synapse formation and maintenance. We observed that dietary fiber preserved synaptic proteins and enhanced hippocampal neurogenesis, likely through improved lipid signaling pathways [5].
AD mice exhibited slightly lower body weights compared to WT mice, consistent with a genotype effect. However, as dietary intervention did not alter body weight and microbiome and metabolic analyses were performed within genotype-matched comparisons, this difference does not affect the interpretation of fiber-specific effects. Together, these results support the concept that FP diets selectively remodel gut microbiota across both phylum and genus levels, thereby promoting a microbial environment favorable for metabolic and anti-inflammatory effects. Consistent with these compositional changes, FP supplementation also enhanced overall microbial diversity, particularly restoring reduced α-diversity in AD mice and driving clear β-diversity separation between FP- and FF-fed groups. However, the roles of individual taxa remain complex and, in many cases, context-dependent and controversial. Thus, while our findings point to reproducible microbial shifts associated with fiber intake, further mechanistic studies are required to delineate the precise functional contributions of these taxa.
Behavioral assessments demonstrated improved cognitive function in FP-fed AD mice. In both the NOR and Y-maze tests, these mice showed enhanced recognition and spatial memory, confirming the protective effects of dietary fiber. In addition to cognitive improvements, our study provides direct evidence that dietary fiber supplementation mitigates hallmark AD pathology in 5xFAD mice. We observed a significant reduction in Aβ deposition and microgliosis, together with restoration of the Aβ42/Aβ40 ratio. Importantly, we adopted an analytical approach in which Aβ42 and Aβ40 staining intensities were normalized to total Aβ load, thereby minimizing variability due to antibody sensitivity differences. Within the context of immunofluorescence histology, this strategy offers an advantage over conventional comparisons of raw signal intensities, as it provides a more internally controlled estimate of the relative burden of Aβ species in defined brain regions [13]. Moreover, detailed morphological analyses of microglia revealed that fiber supplementation attenuated the transition toward an activated amoeboid phenotype, preserving more ramified structures. The observed improvements in cognitive performance align with reduced Aβ deposition and microgliosis in the brain, reinforcing the role of dietary interventions in modulating AD pathology [14]. These results are consistent with studies showing that dietary fibers can reduce amyloid burden and promote synaptic plasticity, potentially through anti-inflammatory and neuroprotection-promoting pathways [15]. Additionally, hippocampal neurogenesis, as evidenced by increased DCX + neurons, was significantly enhanced in FPAD mice.
Neurogenesis is often impaired in AD, and the ability of dietary fiber to promote new neuron formation further supports its neuroprotective potential. Another key finding was improved gut permeability in FP-fed mice, as evidenced by reduced FITC-dextran leakage.
This suggests that dietary fiber helps maintain gut barrier integrity, which is crucial in preventing systemic inflammation—a contributor to AD progression [16,17].
Gut permeability, the “leaky gut, exacerbates neuroinflammation by allowing pro-inflammatory molecules to enter circulation and affect the brain.
This research highlights the intricate relationship between diet, gut microbiota, and brain health. It demonstrates that certain dietary fibers can beneficially modulate lipid metabolism, reduce neuroinflammation, and preserve cognitive function. These findings represent a transformative step forward in understanding how nutrition can be leveraged to prevent or slow the progression of neurodegenerative diseases like Alzheimer’s and Parkinson’s.
References
- Zhao, Y., Taylor, C. M., Lukiw, W. J. & Bazan, N. G. Dietary Fiber Modulates Gut Microbiota and Lipid Metabolism Ameliorating Alzheimer’s Pathology in 5xFAD Mice. Scientific Reports [In Press] (2025).
- Barbaro, M. R. et al. Lactiplantibacillus plantarum (CECT7484 and CECT7485) and Pedioccoccus acidilactici (CECT7483) enhance actin cytoskeleton and CYP1A1 expression restoring epithelial permeability alterations induced by irritable bowel syndrome mediators. Gut Microbes 17, 2452235 (2025).
- Bruce-Keller, A. J. et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 77, 607–615 (2015).
- Saleh, Z. M. et al. Limosilactobacillus reuteri ameliorates maternal separation stress in newborn mice and alters subsequent adult behaviour. Benef Microbes 16, 221–235 (2024).
- Bazan, N. G., Molina, M. F. & Gordon, W. C. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu. Rev. Nutr. 31, 321–351 (2011).
- Emre, C. et al. Intranasal delivery of pro-resolving lipid mediators rescues memory and gamma oscillation impairment in AppNL-G-F/NL-G-F mice. Commun Biol 5, 245 (2022).
- Wang, Y. et al. Maresin 1 attenuates pro-inflammatory activation induced by β-amyloid and stimulates its uptake. J Cell Mol Med 25, 434–447 (2021).
- Do, K. V. et al. Cerebrospinal Fluid Profile of Lipid Mediators in Alzheimer’s Disease. Cell Mol Neurobiol 43, 797–811 (2023).
- Do, K. V. et al. Elovanoids counteract oligomeric β-amyloid-induced gene expression and protect photoreceptors. Proc Natl Acad Sci U S A 116, 24317–24325 (2019).
- Bazan, N. G. Brain Aging and Resilience: Exploring the Adaptability of the Human Brain in the Face of Aging and Adverse Conditions. (Springer, 2025). doi:10.1007/978-3-031-94431-4.
- Lukiw, W. J. et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest 115, 2774–2783 (2005).
- Zhao, Y. et al. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARγ-mediated mechanisms in Alzheimer’s disease models. PLoS ONE 6, e15816 (2011).
- Andersson, E. et al. Soluble cerebral Aβ protofibrils link Aβ plaque pathology to changes in CSF Aβ42/Aβ40 ratios, neurofilament light and tau in Alzheimer’s disease model mice. Nat Aging 5, 366–375 (2025).
- Heneka, M. T. et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14, 388–405 (2015).
- Frausto, D. M., Forsyth, C. B., Keshavarzian, A. & Voigt, R. M. Dietary Regulation of Gut-Brain Axis in Alzheimer’s Disease: Importance of Microbiota Metabolites. Front Neurosci 15, 736814 (2021).
- He, J. et al. Intestinal changes in permeability, tight junction and mucin synthesis in a mouse model of Alzheimer’s disease. Int J Mol Med 52, 113 (2023).
- Pellegrini, C. et al. The intestinal barrier in disorders of the central nervous system. Lancet Gastroenterol Hepatol 8, 66–80 (2023).
Follow the Topic
-
Scientific Reports

An open access journal publishing original research from across all areas of the natural sciences, psychology, medicine and engineering.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Reproductive Health
Publishing Model: Hybrid
Deadline: Mar 30, 2026
Women’s Health
Publishing Model: Open Access
Deadline: Feb 28, 2026
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in