Olanzapine, risperidone and ziprasidone differently affect lysosomal function and autophagy, reflecting their different metabolic risk in patients
Published in General & Internal Medicine and Anatomy & Physiology
Psychiatrists know that antipsychotic drugs cause weight gain. Not all know that antipsychotics also cause alterations in glycaemic and lipidic parameters, and that these issues could be avoided. This happens because translational research on the metabolic adverse effects of antipsychotics is still needed, in order to elucidate why and to characterise how antipsychotics cause metabolic alterations. Such knowledge can be propaedeutic to direct the search for treatment options alternative to current antipsychotics, as well as to find possible pharmacological or nutritional countermeasures to metabolic adverse effects, to avoid an iatrogenic metabolic syndrome, which currently occurs usually after prolonged antipsychotic use [J Am Acad Child Adolesc Psychiatry. 2023 Jun 23:S0890-8567(23)00317-9].
Antipsychotics are known to regulate appetite and satiety, psychopharmacologically and by neuroendocrine mechanisms. Psychopharmacological and neuroendocrine disruptions increase caloric intake and weight gain. However, they do not seem to mediate glycolipidic dysregulations as well: several independent studies observed how glycolipidic dysregulations can arise hours after antipsychotics intake in patients, when feeding and weight are not yet perturbed.
This suggests a different and complementary mechanism of action of antipsychotics, which descends from their biochemical nature of amphiphilic bases. Psychoactive drugs have to be lipophilic, in order to cross the blood-brain barrier, otherwise they would not work. Some may in fact be amphiphilic, having a hydrophilic portion as well. This allows partitioning into lipid or aqueous compartments based only on the ionic charge. At surrounding pH inferior to the drug pKa it will be positively charged, getting pulled out of lipids into aqueous compartments. Most antipsychotics are weak bases and can be protonated only in strongly acidic compartments, such as lysosomes or late endosomes; a few are strong bases and can be protonated also in neutral compartments, such as the cytosol or early endosomes. Previous work showed how the polarizability and pKa of antipsychotics can influence their adverse effect profile [Psychological Medicine. 2022;52(15):3508-3520] and, in this work, we aimed to increase the knowledge of the mechanisms of adverse metabolic action of antipsychotics as amphiphilic bases. We used, alongside other positive controls, the non-antipsychotic amphiphilic weak base U18666A, which was previously used to establish an experimental model of Niemann Pick Type C disease, validating its damaging potential on all performed assays.
We found differences among antipsychotics in vitro concerning SREBP-dependent transcription, lysosomal function and autophagy, and AMPK activation, while excluding effects mediated by neurotransmitters. We used human hepatoma cells HepG2 and maintained a clinically relevant glucose concentration (100 mg/dL) with low external cholesterol loads (20% FBS). Interestingly, most previous publications could not observe these differences, which were maintained also when applying non-physiological high glucose conditions.
We observed that olanzapine is highly damaging, risperidone moderately damaging, and ziprasidone is not damaging, in keeping with their metabolic impact in patients.
Olanzapine treatment causes the engulfment with unesterified sterol cargo of lysosomes and endolysosomal structures, leading to enlargement, reducing lysosomal function and inhibiting the autophagic flux. Consequences are the presence of autophagosome-lysosome fusion defects with the accumulation of autophagosomes and amphisomes (endosomes fused with autophagosomes), and the increase in the overall SREBP-dependent transcription with final accumulation of lipid droplets and cholesterol precursors, possibly due to the mechanism previously described as “activation through inhibition”.
Risperidone treatment had no significant effect on lysosomes and on autophagy; it only affected the transcription of genes downstream SREBP1 and led to the accumulation of lipid droplets, suggesting that the interference with lysosomal function can be an aetiopathogenic mechanism, but may be not required for the induction of a metabolic transcriptional response. Moreover, the finding that risperidone induces downstream genes without activating SREBP1 suggests the involvement of other transcription factors that could be activated by specific sterol intermediates, that are increased by risperidone as by-products of inhibited cholesterol biosynthesis.
Ziprasidone, in line with its clinical effect of weight reduction and the reported absence of glycolipid alterations, had a transcriptional repression effect on SREBP1- and SREBP2-target genes, reduced triglyceride levels, did not affect cholesterol, lysosomal morphology and function, and did not alter the autophagic flux.
Moreover, we showed that AMPK inhibition or silencing is sufficient, but not necessary, to induce a metabolic phenotype similar to the one caused by olanzapine. Considering this important role of AMPK, we tried to revert antipsychotic-induced metabolic effects by co-treatment with metformin. The rationale for this choice is also that the oral hypoglycaemic drug metformin is efficacious for the treatment of obesity consequent to type 2 diabetes, but there is no indication of metformin efficacy on the lipid and cholesterol profiles of patients, which are often altered by antipsychotics use. Of note, metformin has been used in clinical trials to treat or prevent dysmetabolism consequent to antipsychotic use, with good and expected effect on weight loss and glycaemic profiles, and a scarce effect on dyslipidaemia.
Metformin concomitant treatment reduced the transcription downstream SREBP1, increased the autophagic flux and reduced lipid droplets accumulation, it increased the production of bile acids and reduced unesterified cholesterol contents and lysosomal size. Effects were sufficient to revert most of the metabolic damage induced by olanzapine and risperidone; metformin did not rescue the increased SREBP2-dependent transcription, probably due to the molecular mimicry property of antipsychotics, which keeps active the low cholesterol-dependent trigger for SREBPs cleavage.
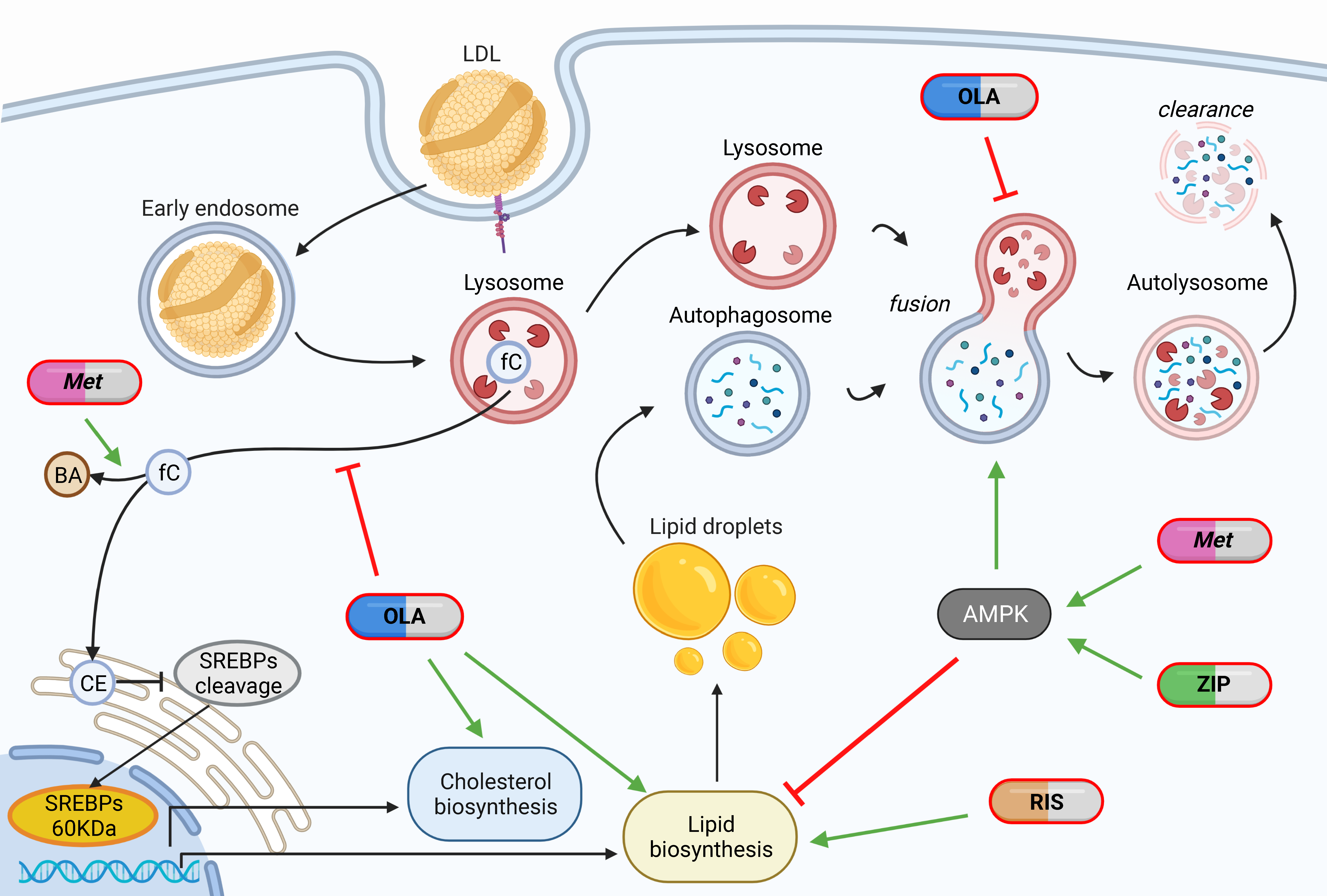
In conclusion, marked differences were found between antipsychotics: olanzapine seems to act through the disruption of lysosomal function and autophagy impairment, causing both accumulation of endocited cholesterol and synthesised lipids; risperidone seems to act through a different yet unknown mechanism of action, causing accumulation of synthesised lipids; ziprasidone seems to act through AMPK activation, causing no accumulations. Metformin seems to have a manifold effect dependent on AMPK activation: it reduces lipid synthesis, ameliorates lysosomal enlargement and cholesterol accumulation, increases the transcription of LDL receptors, induces autophagy, leading to increased clearance of lipid droplets, and possibly also of cholesterol, increases conversion of cholesterol into bile acids.
Combined olanzapine + metformin treatment results in a still overactive and inhibited cholesterol biosynthesis, whereas risperidone + metformin treatment results in an unclear effect on autophagy, aspects that require clarification. However, the rescue of other metabolic phenotypes supports metformin as a promising treatment for olanzapine- and risperidone-induced metabolic disorders.
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Related Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Feb 28, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in