Omicron variant neutralizing antibodies following BNT162b2 BA.4/5 versus mRNA-1273 BA.1 bivalent vaccination in patients with end-stage kidney disease
Published in Biomedical Research

Due to the ongoing evolution of SARS-CoV-2 variants of concern, bivalent COVID-19 vaccines targeting B.1.1.529 (Omicron) subvariants were approved. In Canada, the mRNA-1273 (Moderna) BA.1 COVID-19 vaccine was the first bivalent vaccine approved on September 1, 2022 although by this time, BA.5 had a higher prevalence in the community. This was followed by the approval of the BNT162b2 (Pfizer-BioNTech) BA.4/5 COVID-19 vaccine on October 7, 2022. Patients with chronic kidney disease including those on dialysis or with a kidney transplant are a vulnerable population at higher risk for severe COVID-19 and have impaired humoral response to vaccination.1-3 The emergence of Omicron BQ.1.1 and XBB.1.5 raised further concern that these subvariants might have immune-evasive potential.4,5 Given that the mRNA-1273 bivalent vaccine targeted BA.1 while BNT162b2 targeted BA.4/5, we decided to compare differences in neutralizing antibody levels against BA.1, BA.5, BQ.1.1, and XBB.1.5 with these two bivalent vaccines.
We studied 98 adults on maintenance hemodialysis or with a kidney transplant who had received either bivalent vaccine between July 25 and November 30, 2022. We measured their SARS-CoV-2 neutralizing antibodies prior to and 1 month after bivalent vaccination using a spiked-pseudotyped lentiviral neutralization assay (information on the spike protein constructs for different subvariants is available at http://nbcc.lunenfeld.ca/resources).
In our cohort, the median age was 69 years, 85% were dialysis recipients, and 15% had a kidney transplant. Due to the timing of vaccine availability, the majority (73%) received the mRNA-1273 BA.1 vaccine, while 27% received the BNT162b2 BA.4/5 vaccine. We also tested for anti-nucleocapsid seropositivity as a marker of prior COVID-19 infection: 41% (40/98) were seropositive for anti-nucleocapsid. During the study, no new COVID-19 infections occurred.
What did we find? Neutralization against Omicron BA.1, BA.5, BQ.1.1, and XBB.1.5 increased by approximately 8-fold one month following bivalent vaccination (Fig.1).

Fig. 1: Neutralizing capacity against SARS-CoV-2 Omicron subvariants prior to and 1 month following bivalent mRNA COVID-19 vaccination.
In comparison to wild-type (D614G), neutralizing antibodies against Omicron-specific variants were 7.3-fold lower against BA.1, 8.3-fold lower against BA.5, 45.8-fold lower against BQ.1.1, and 48.2-fold lower against XBB.1.5. While absolute neutralizing antibody levels were higher in those receiving the BNT162b2 vaccine, one month following bivalent vaccination, these differences were not statistically different by vaccine type for wild-type (D614G) (P=0.48), BA.1 (P=0.21), BA.5 (P=0.07), BQ.1.1 (P=0.10), or XBB.1.5 (P=0.10) (Fig. 2a).
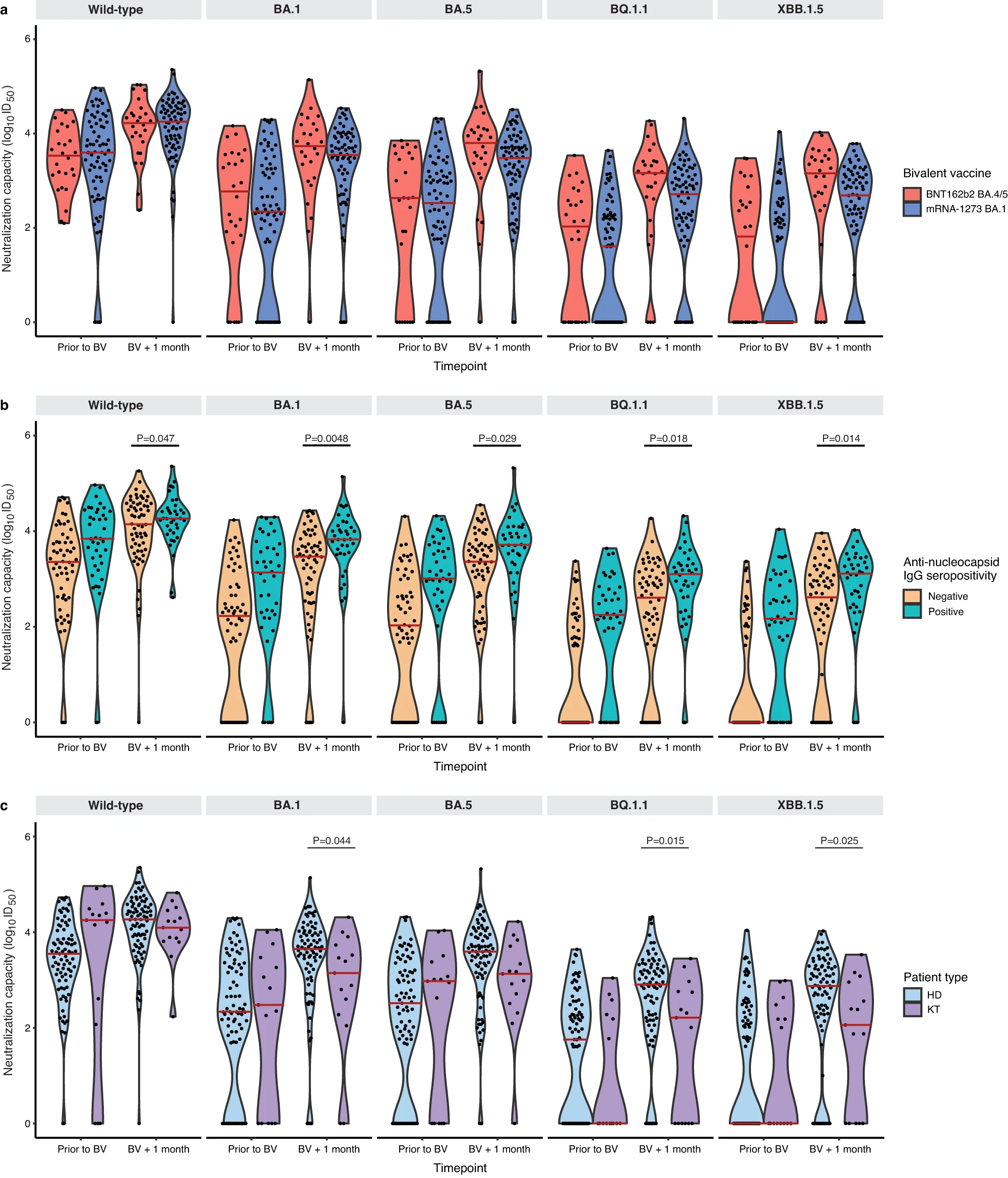
Fig. 2: Neutralizing antibodies against wild-type, BA.1, BA.5, BQ.1.1, and XBB.1.5 prior to and following bivalent vaccination.
Consistent with other studies which show that hybrid immunity (vaccination plus infection) offers superior immunity6, anti-nucleocapsid seropositivity was associated with higher neutralizing antibodies for all Omicron subvariants: BA.1 (P=0.0048), BA.5 (P=0.029), BQ.1.1 (P=0.018), and XBB.1.5 (P=0.014) (Fig. 2b). Interestingly, there was no difference in antibody levels between those who had received the bivalent vaccine as the fourth dose (n=8) versus the fifth dose (n=90). Kidney transplant recipients (n=15) had significantly lower absolute neutralizing antibody levels than hemodialysis patients (n=83) after vaccination against BA.1 (P=0.044), BQ.1.1 (P=0.015), and XBB.1.5 (P=0.025), but not wild-type (P=0.42) or BA.5 (P=0.063) (Fig. 2c). This is not surprising, given that kidney transplant recipients were all receiving a triple immunosuppressive regimen (corticosteroids, anti-metabolite, and a calcineurin inhibitor) which would be expected to attenuate the humoral response.
This is the first study in patients with kidney disease to compare neutralizing antibody response against Omicron subvariants with bivalent vaccines targeting BA.1 versus BA.4/5. We were also able to account for potential confounders including prior COVID-19, vaccine doses in our analysis. We also conducted several sensitivity analyses which did not change our findings.
Overall, our findings provide reassurance to individuals who opted to receive the mRNA-1273 BA.1 vaccine (because it was the first available bivalent vaccine in Canada) as the neutralizing antibody response against all Omicron subvariants evaluated did not differ by vaccine type. This suggests that variant modified bivalent COVID-19 vaccines offer cross-protection even when the circulating Omicron subvariant has already diverged antigenically.
In summary, we found that both BNT162b2 (BA.4/5) and mRNA-1273 (BA.1) bivalent vaccines elicited a similar neutralizing antibody response against Omicron subvariants including BQ.1.1 and XBB.1.5. Nevertheless, all patients in this study will be encouraged to receive vaccination with the recently approved monovalent XBB.1.5 COVID-19 vaccines.
References:
- Krueger, K.M., Halasa, N. & Ison, M.G. SARS-CoV-2 Vaccine in Dialysis Patients: Time for a Boost? Am J Kidney Dis 79, 162-163 (2022).
- Cai, R., et al. Mortality in chronic kidney disease patients with COVID-19: a systematic review and meta-analysis. Int Urol Nephrol 53, 1623-1629 (2021).
- Taji, L., et al. COVID-19 in patients undergoing long-term dialysis in Ontario. CMAJ 193, E278-E284 (2021).
- Miller, J., et al. Substantial Neutralization Escape by SARS-CoV-2 Omicron Variants BQ.1.1 and XBB.1. N Engl J Med (2023).
- Uraki, R., et al. Antiviral and bivalent vaccine efficacy against an omicron XBB.1.5 isolate. Lancet Infect Dis 23, 402-403 (2023).
- Bates, T.A., et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol 7, eabn8014 (2022).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in