On Colorectal Cancer: Can Blocking DNA Repair Improve Outcomes After HIPEC? Insights From Organoid Models
Published in Cancer
For patients with colorectal cancer (CRC) that has spread to the peritoneal cavity (the space in which the abdominal organs lie), treatment options are limited. The only available cure is cytoreductive surgery (CRS) followed by hyperthermic intraperitoneal chemotherapy (HIPEC), a procedure in which heated chemotherapy is circulated directly in the abdomen to destroy remaining tumor cells.
Although CRS-HIPEC can be life-extending for a select group of patients, it comes with a sobering reality: around 80% of patients still experience tumor recurrence. Understanding why recurrence is so common and how we can prevent it remains one of the biggest challenges in this field.
In our study, we set out to test a simple yet powerful idea: what if we could make tumor cells less able to repair the DNA damage caused by chemotherapy? The cancer cells seem to survive the chemotherapy, but more chemotherapy would be too toxic for the patients. Perhaps another drug can give chemotherapy a helping hand by blocking the survival mechanism of the cells. Maybe in this way we can prevent the recurrence.
To explore this, we turned to patient-derived organoids, a cutting-edge laboratory model that allows us to study the behavior of individual patients’ tumors outside the body. Using organoids created from peritoneal metastases (PM), we evaluated whether blocking the tumor’s DNA Damage Response (DDR) could stop cancer regrowth after HIPEC-like treatment.
Below, I explain what we did, what we found, and why this might matter for future therapies.
Why DNA Repair Matters in Chemotherapy
Many chemotherapies, including those used during HIPEC, work by damaging DNA. If cells accumulate too much DNA damage, they die. Cancer cells, however, can often survive chemotherapy because they activate the DNA Damage Response, a network of proteins that detects damage, pauses cell division, and helps repair the DNA (Figure 1). Several drugs that inhibit key players of the DDR (such as ATR, CHK1, and WEE1) are now in clinical development.
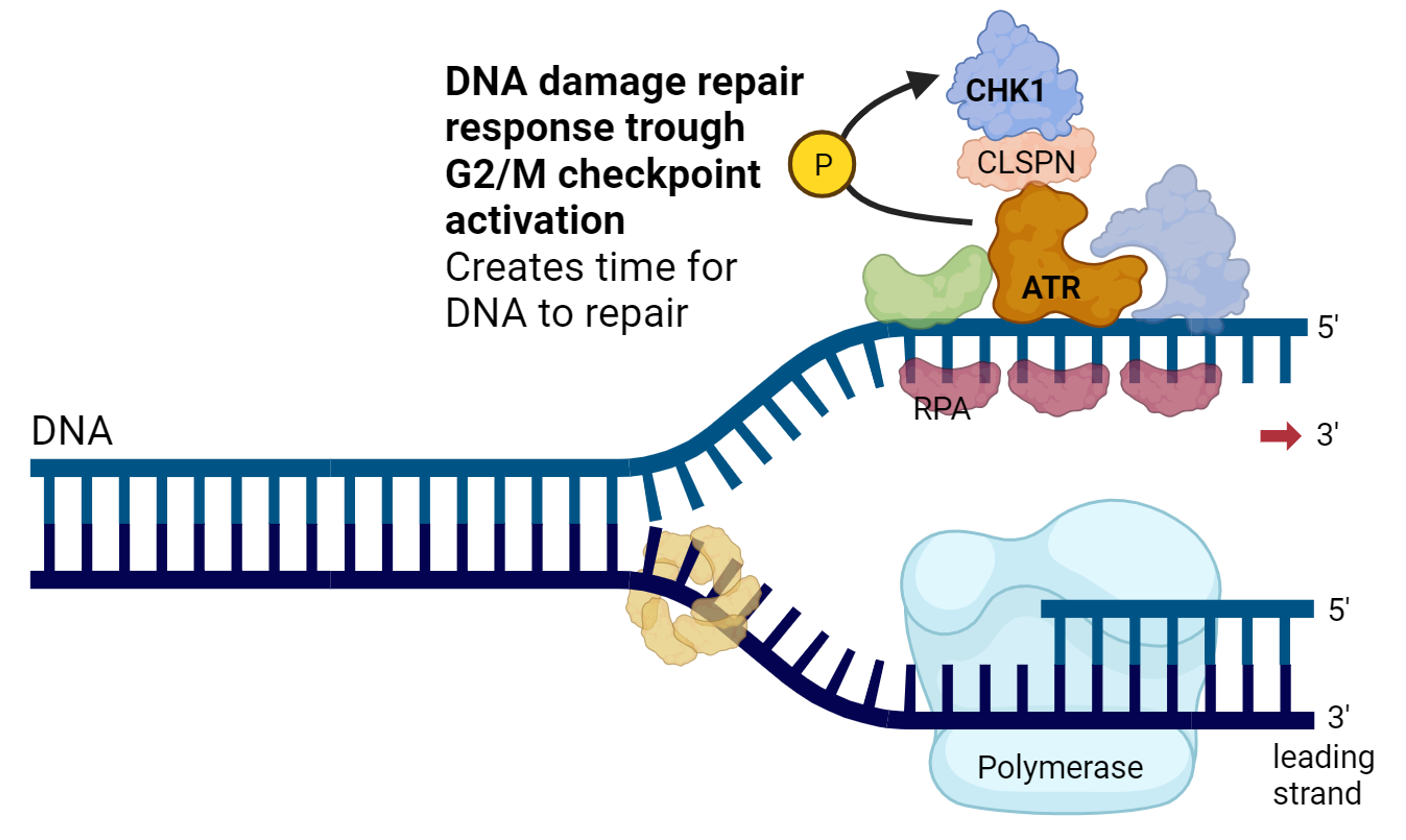
Figure 1. The DNA Damage Response machinery of the cell. DNA Damage triggers activation of ATR and CHK1, allowing the cell to pause its cell cycle. This gives the cell time to repair its DNA. Useful for the survival of healthy cells, also what cancer cells use to survive chemotherapy.
Our hypothesis was straightforward: If we combine chemotherapy with DDR inhibitors, cancer cells might sustain fatal levels of DNA damage and be unable to grow back.
Organoids: A Model for Studying Recurrence
Traditional cell lines often fail to reflect the complexity of human tumors and the diversity of patients. To overcome this, we used peritoneal metastasis-derived organoids (PMDOs); miniature 3D tumor cultures grown from patient samples.
We tested organoids from ten patients using a range of DNA damage response inhibitors alongside chemotherapies that are used for intraperitoneal treatment in the clinic. By combining these drugs and assessing the effects with microscopy, viability assays, and protein analysis, we focused on two questions: how effectively the treatments damaged tumor cells, and whether the organoids were able to regrow afterward: a measure of recurrence.
DDR Inhibition synergizes with MMC to overcome PMDO outgrowth
The results were clear: all ten PMDO models could survive treatment with chemotherapy alone. The organoids initially decreased in size but subsequently recovered, reflecting the clinical challenge of resistance and recurrence. However, when DDR inhibitors were added to MMC, regrowth was consistently prevented in all models (Figure 2).
Figure 2. Example of synergy between three-day treatment with MMC treatment and with various DDR inhibitors in PMDO02-1. Where organoids continue to grow after MMC treatment alone, co-treatment with either one of the DDR inhibitors prevents this, eliminating all cancer cell growth.
On a molecular level, berzosertib prevented activation of CHK1, a downstream marker of the ATR pathway. This meant the tumor cells were unable to pause the cell cycle and repair DNA damage caused by MMC. As a result, DNA damage accumulated beyond repair, and the organoids were eradicated. Although the combinations with oxaliplatin and irinotecan showed partial improvement, they were far less consistent than MMC, likely due to differences in DNA-damaging mechanisms.
“Adjuvant” DDR Inhibition prevents recurrence after HIPEC
Next, we set up to mimic the clinical setting of HIPEC procedures. We wondered whether giving DDR inhibitors after HIPEC-like treatment might also be effective Hence, we exposed organoids to MMC in a simulated HIPEC procedure (90-minute MMC treatment at 42°C), followed by a three-day treatment with DDR inhibitors alone.
Indeed, this approach was just as effective as simultaneous combination treatment, even at 37°C. Every organoid treated with MMC followed by the DDR inhibitor showed a significant inhibition of regrowth. These results suggest that DDR inhibitors might be valuable not only during MMC treatment but also as an adjuvant therapy after HIPEC, a possibility that could be more practical and safer for patients.
In summary, our results show that peritoneal metastasis-derived organoids can be completely eradicated when MMC treatment is followed by inhibition of ATR or related DNA damage response kinases. These findings suggest that DDR inhibitors may have potential as adjuvant therapy to reduce recurrence after CRS-HIPEC and warrant further investigation in clinical settings
Limitations and Next Steps
While our results are encouraging, they are still preclinical. Key questions to be explored in further research include: Will DDR inhibitors be safe and tolerable when used shortly after HIPEC? How long should treatment last, and at what dose? And which drug will be best? Careful clinical testing will tell whether DDR inhibition can benefit PM-CRC patients treated with MMC in the clinic.
A Step Toward Reducing Recurrence in Peritoneal Metastases
CRS-HIPEC remains one of the most aggressive treatments in cancer care, yet recurrence rates are dishearteningly high. Our study suggests that this may not be an unsolvable problem. By pairing established chemotherapy with emerging DDR-targeting drugs, we may be able to strike tumor cells harder, enough to really wipe them out.
While much more research is needed, this work provides a foundation for rethinking how we approach recurrence prevention in peritoneal metastases since monotherapies do not seem sufficient.
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.
Ask the Editor – Inflammation, Metastasis, Cancer Microenvironment and Tumour Immunology
Got a question for the editor about inflammation, metastasis, or tumour immunology? Ask it here!
Continue reading announcement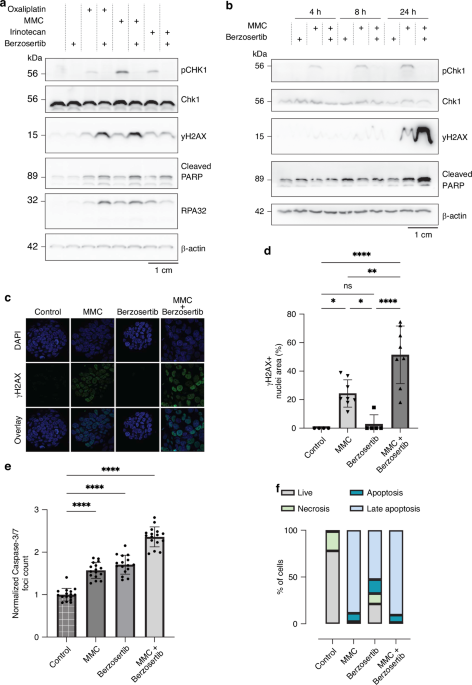


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in