Origin of life: non-linear research path, for a non-linear phenomenon
Published in Chemistry
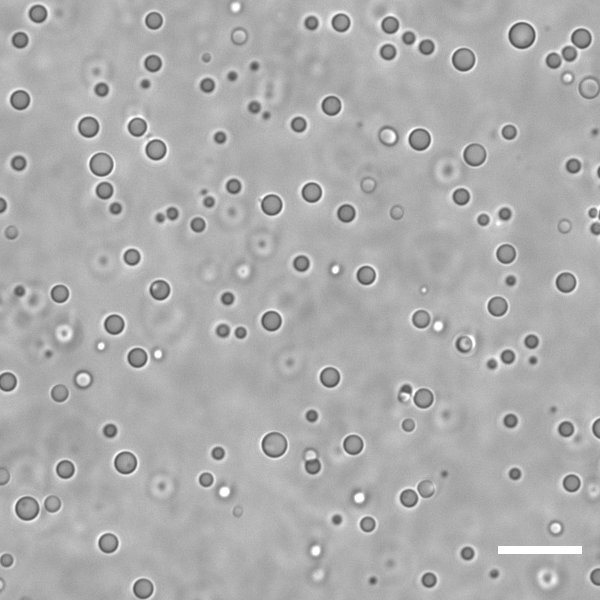
When Fatma and I began to work with primitive coacervates, as the first ones to do so in the group, there were many models to choose from, like the peptide synthon found in 2021 by Abbas et al, the dipeptide shown by Cao et al in 2023, or the metabolites described by Smokers, van Haren et al in 2022. We questioned if such models, although extremely prebiotically plausible, were reflective of the origin of life scenario we were trying to evoke - more aligned with a systems chemistry approach. We noticed an important blind spot: while there are common synthetic routes for amino acids and nucleotides, or for peptides and nucleic acids, coacervates composed of both peptides and nucleic acids (called heterotypic) are often only considered at later stages of the emergence of life, when polypeptides and ribozymes are present.
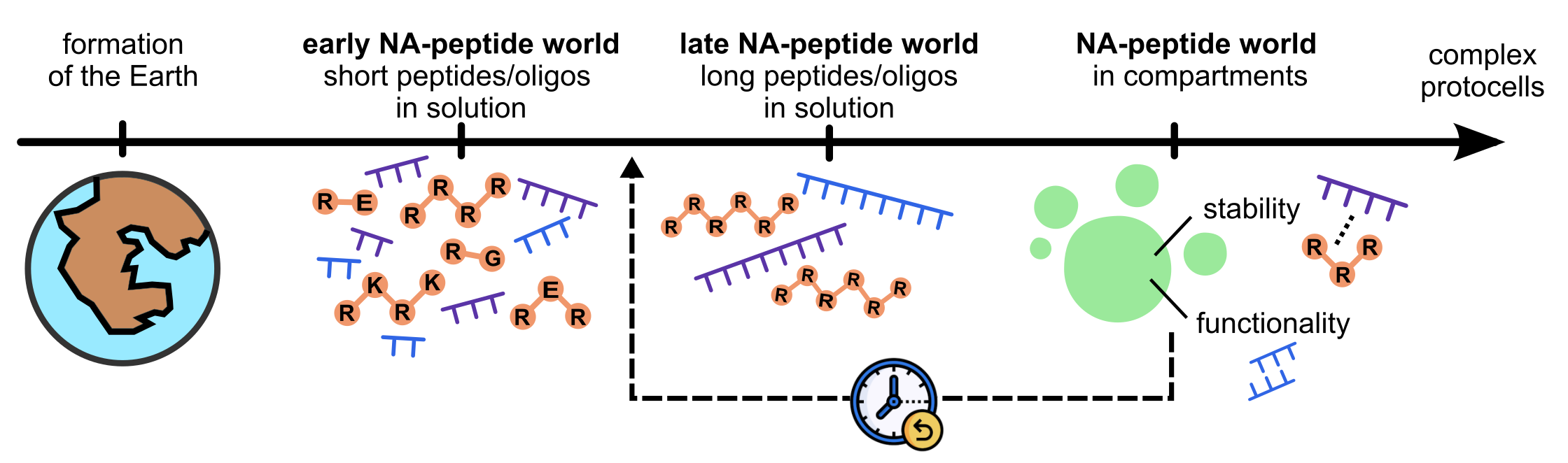
Figure 1. Our envisioned timeline for the early stages of life, adapted from Joyce, Nature (2002) and now anticipating the emergence of compartments to an early nucleic acid-peptide world.
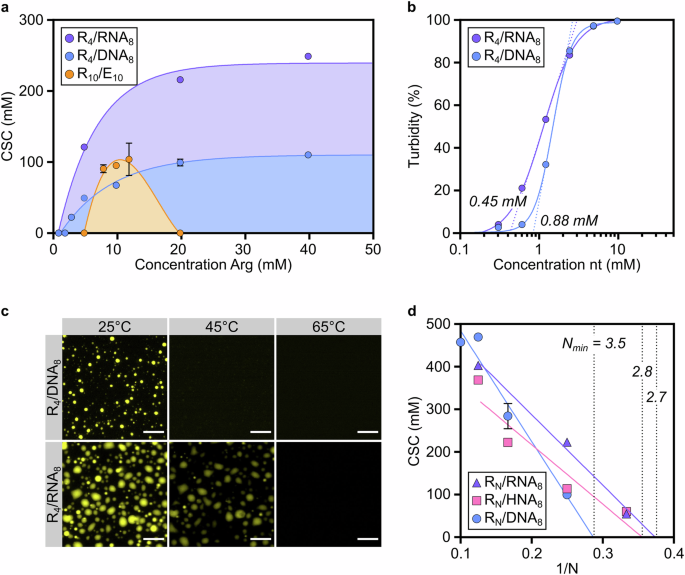
Work from Christine Keating, Dora Tang and Phil Bevilacqua indicated that arginine-rich peptides of no more than 10 amino acids were a good starting point. We selected R10, R8, R6, R4 and so on, and DNA of 40, then 32, then 20, then 12, then 8 nucleobases. We found that we could go as minimalistic as R4/DNA8 and still obtain coacervate droplets of reasonable stability (up to ca. 100 mM NaCl), as long as the mixture would have an excess of arginine, compared to nucleobase concentration. This was unlike homotypic coacervates, which are most stable around charge unity, but not completely surprising. There are similar reports in the literature for PLL/ATP and spermine/polyU mixtures, with mostly intuitive explanations.
That is when we reached out to Rosana Collepardo's group in Cambridge, and asked if it would be possible to understand a bit more in depth why the excess arginine was needed for stability in the R4/DNA8 case - out of genuine curiosity (side quest #1). With her group, we found out that the simulations community was heavily focused on condensates made of dsDNA or ssRNA, but not ssDNA. While her group tuned the parameters of their force fields to simulate our mixtures at an atomistic level, we went on to side quest #2.
The focus on RNA over DNA is not just specific to computational works, but also present in prebiotic chemistry. There is a quasi-consensus that RNA predated DNA. Most of the time when prebiotic chemists use DNA instead of RNA, it is for budget reasons; DNA is significantly cheaper, and it makes sense to use it as a proxy for RNA in preliminary experiments - especially for single-stranded DNA, the difference should be minimal. We decided we were ready to upgrade our minimalistic coacervates to peptide/RNA ones. To our surprise, the additional -OH group led to an almost doubling of stability, and also meant that peptide lengths for which we would not see phase separation with DNA were now demixing in the presence of RNA. While it is easy to imagine that an extra -OH group does indeed lower the interaction energy of the peptide/oligo complexes, it was difficult to explain the magnitude of the effect. So we came back to Rosana's group for an update on the atomistic model, and to bring this new question about the difference between DNA and RNA, which they were later able to explain in detail in terms of DNA compaction.
When we started to use RNA with our arginine-rich peptides, there was an obvious follow up question. In 2016, Szostak's group had reported that oligoarginines assist non-enzymatic RNA replication by forming coacervates; but those findings could not be reproduced and the group retracted the paper. As a 1st year PhD working on coacervates as protocells back in 2017, I vividly remember the impact of the retraction - not because it was not the right thing to do in that case, but because the community had perceived it as a major breakthrough, the conciliation of peptides with RNA replication chemistry. In a meeting with Derek O'Flaherty about a different project, came side quest #3: would our minimalistic R4/DNA8 or R4/RNA8 support the elusive non-enzymatic RNA replication?
Side quest #3 turned out to be the most time-consuming. Derek and his group in Guelph are experts in primer extension, from synthesizing the activated monomer to quantifying reaction yields. But they had never carried on the reaction in the presence of coacervates, that is, in an emulsion phase, or observed their reaction under the microscope. While coacervates spontaneously (easily) form upon mixing, it is always good to confirm the morphology of the droplets under a microscope. In a truly collaborative effort, in our end in Strasburg we screened coacervate compositions that would tolerate the components of the primer extension reaction and the best way to homogenize the mixture before analysing it. After many calls, pictures of micro-pellets at the bottom of micro-centrifuge tubes exchanged, double and triple checks, we began to see trends in the results coming from Guelph. Trends similar to what Dr Roger Rubio-Sánchez was obtaining performing FRAP in our coacervates in Cambridge. Peptides like R4 and R6 on their own would inhibit RNA elongation - not very different from what Bevilacqua showed for R10. It was only when the reaction mixture was a mere guest in peptide/oligonucleotide coacervates that we could finally observe the significant yields and the effects described in our paper - and that explain why primer extension in peptide/RNA condensates had been such an elusive process.
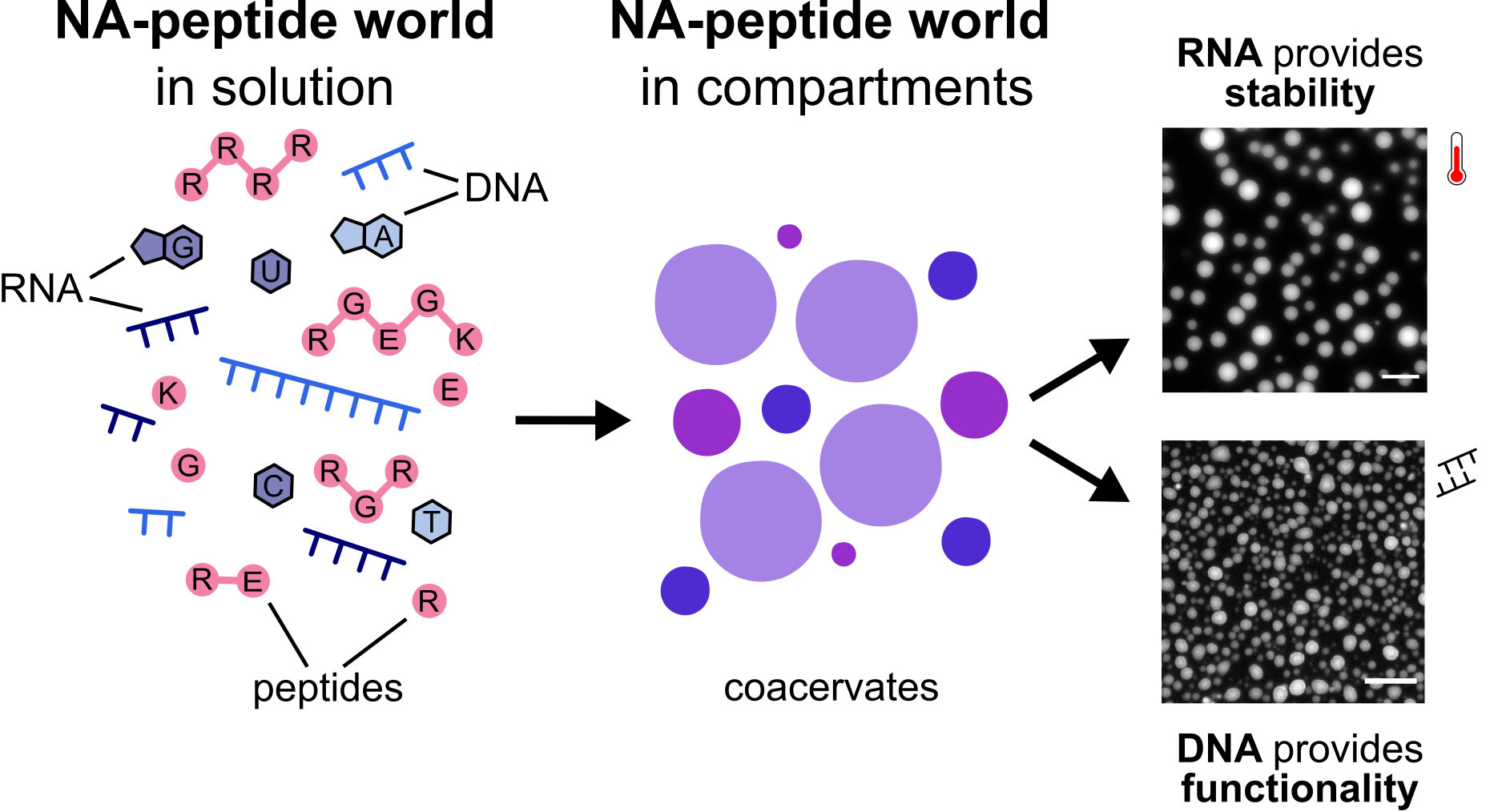
Figure 2. Summary of our results: at early stages of a NA-peptide world, when peptide trimers and nucleic acid octamers would be present, coacervation could have been inevitable. RNA significantly increases the tendency for and the stability of coacervates to salt and heat stress, while DNA improves the mobility of RNA primers in the droplets, and ultimately, reactivity (functionality).
These are just some of our side quests. There were many others; a geographical diversion, when our PI was offered a position at the Department of Biochemistry in Cambridge, and we had the opportunity to join her. While it meant pausing our experiments right at the climax of the primer extension results, it surely felt like a promotion for prebiotic chemists to now be working at an extant-life department. Some scientific divergences - it was not always easy to decide how to communicate our findings, in parts or altogether. We were in fact privileged to be able to make room for all these questions, in a true fundamental science quest fashion. Much like an RNA primer in a network of peptide-oligonucleotide interactions, our collaborations kept us active and stable throughout hardships, and enabled us to come back from each and every digression more knowledgeable than before.
Written by Karina Nakashima and Fatma Zohra Mihoubi
References
Abbas, M., Lipiński, W.P., Nakashima, K.K. et al. A short peptide synthon for liquid–liquid phase separation. Nat. Chem. 13, 1046–1054 (2021). https://doi.org/10.1038/s41557-021-00788-x
Cao, S., Ivanov, T., Heuer, J. et al. Dipeptide coacervates as artificial membraneless organelles for bioorthogonal catalysis. Nat Commun 15, 39 (2024). https://doi.org/10.1038/s41467-023-44278-9
I. B. A. Smokers, M. H. I. van Haren, T. Lu, E. Spruijt. Complex Coacervation and Compartmentalized Conversion of Prebiotically Relevant Metabolites. ChemSystemsChem 2022, 4, e202200004. https://doi.org/10.1002/syst.202200004
Cakmak, F.P., Choi, S., Meyer, M.O. et al. Prebiotically-relevant low polyion multivalency can improve functionality of membraneless compartments. Nat Commun 11, 5949 (2020). https://doi.org/10.1038/s41467-020-19775-w
Iglesias-Artola, J.M., Drobot, B., Kar, M. et al. Charge-density reduction promotes ribozyme activity in RNA–peptide coacervates via RNA fluidization and magnesium partitioning. Nat. Chem. 14, 407–416 (2022). https://doi.org/10.1038/s41557-022-00890-8
Poudyal, R.R., Guth-Metzler, R.M., Veenis, A.J. et al. Template-directed RNA polymerization and enhanced ribozyme catalysis inside membraneless compartments formed by coacervates. Nat Commun 10, 490 (2019). https://doi.org/10.1038/s41467-019-08353-4
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in