Osteopontin: a new therapeutic alarm clock in breast cancer recurrence
Published in Biomedical Research
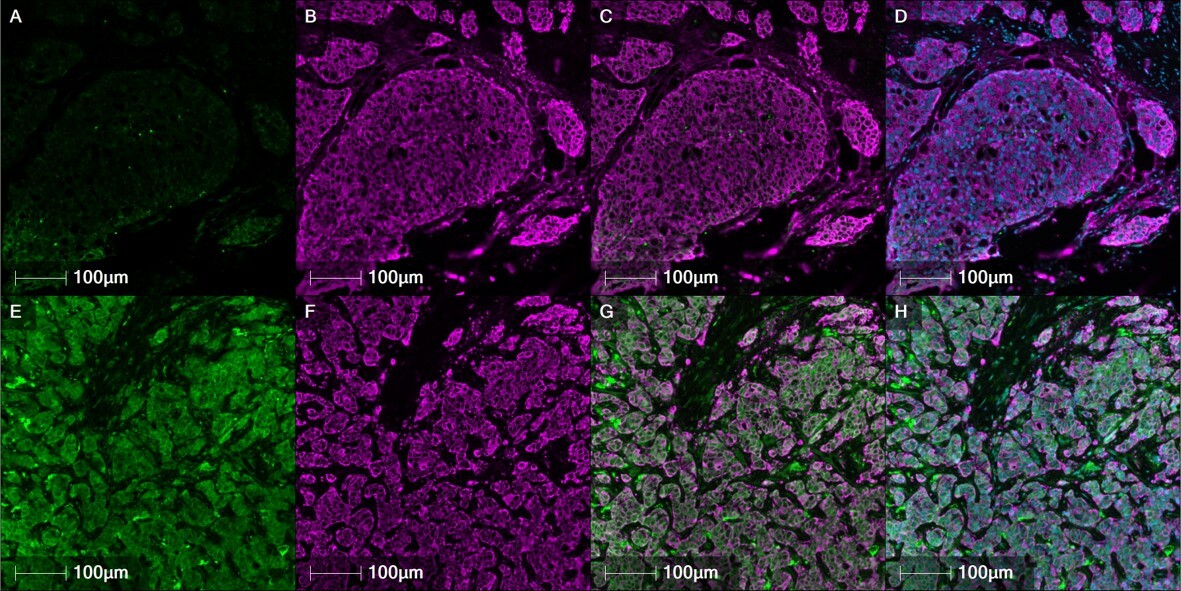
The Challenge: Breast cancer recurrence and metastasis
The relatively high survival rate for breast cancer patients can be quite misleading. For too many, the battle doesn’t end with the ringing of the “cancer bell.” Local and metastatic recurrent breast cancers often foster therapy resistance, and eventually cause disease lethality. They arise from residual cancer cells when their intrinsic and environmental alarm clocks strike twelve.
The Alarm: Osteopontin, a matrix protein and cytokine
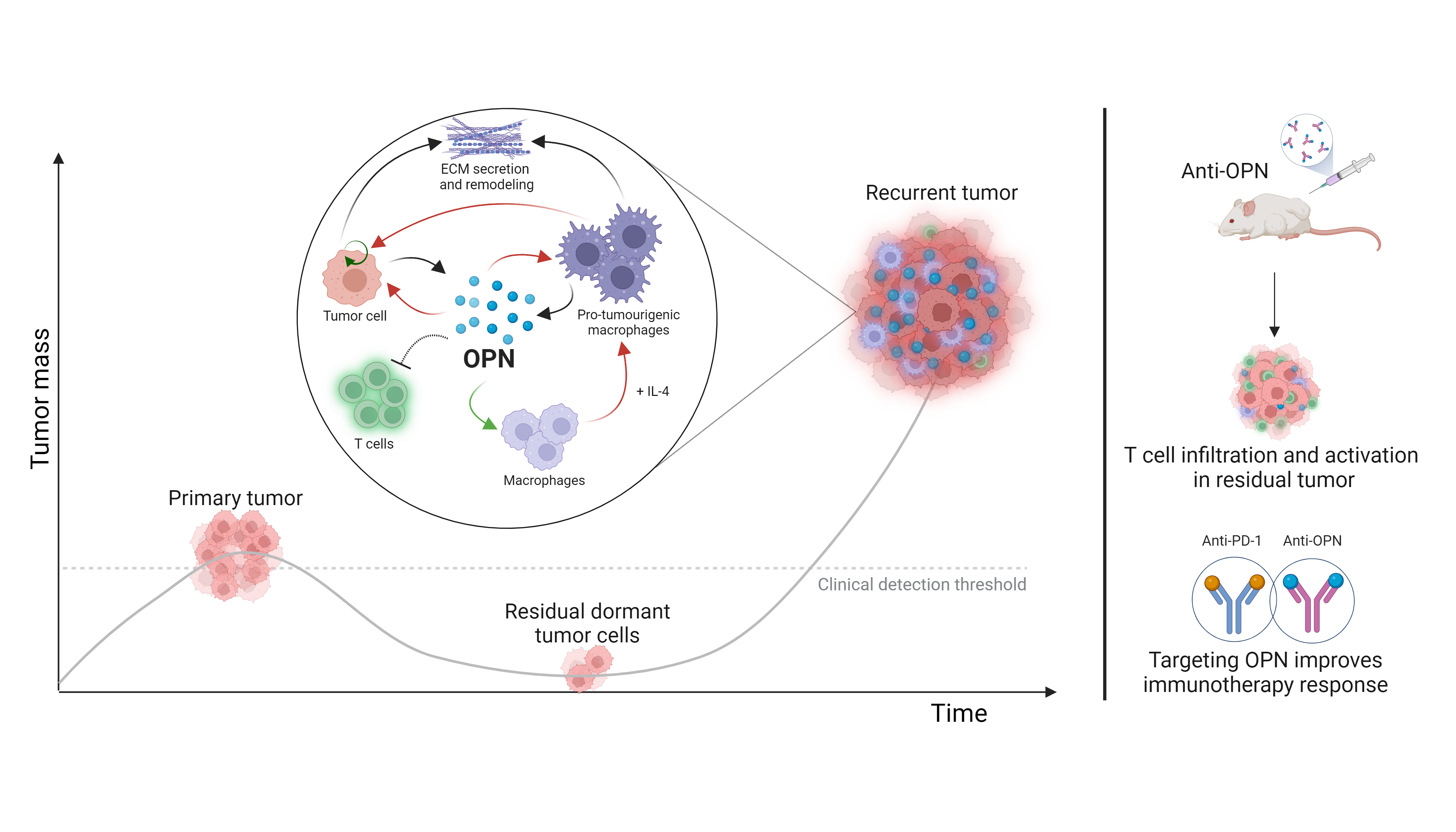
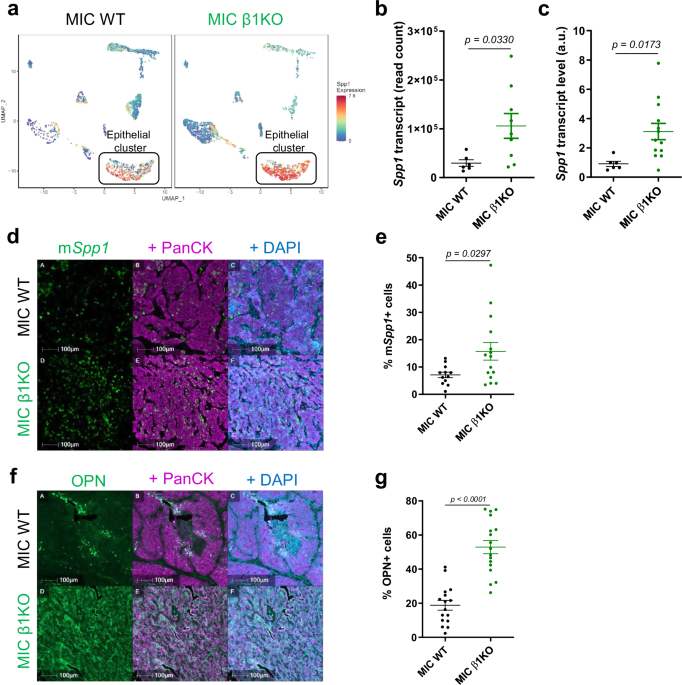
In our recent study, we pinpoint osteopontin, an extracellular matrix protein and secreted cytokine, to be a rather loud alarm that drives breast cancer recurrence. At once, osteopontin serves both autocrine and paracrine roles. It directly promotes cancer cell proliferation and tumor growth, specifically in the recurrent stage, and acts on nearby macrophages to favor recurrence.
The Mechanisms: How osteopontin wakes up a tumor
Our work untangled the many ways by which osteopontin contributes to cancer recurrence. As a direct target of the transcription factor Stat3, we saw elevated osteopontin at both RNA and protein levels in a pre-clinical model of recurrent breast cancer. To further understand its role, we introduced exogenous osteopontin protein in mice, the tumors of which grew significantly faster than control mice.
As with all solid tumors, cancer cells don’t live alone. Within the recurrent tumor environment, cancer cells are surrounded by macrophages which are recruited by osteopontin. Once there, they become polarized to a pro-tumorigenic state by osteopontin and IL-4. Altogether, osteopontin’s multifaceted influence is vital for the tumor’s ability to thrive and recur.
The Snooze Button: Unlocking therapeutic potential
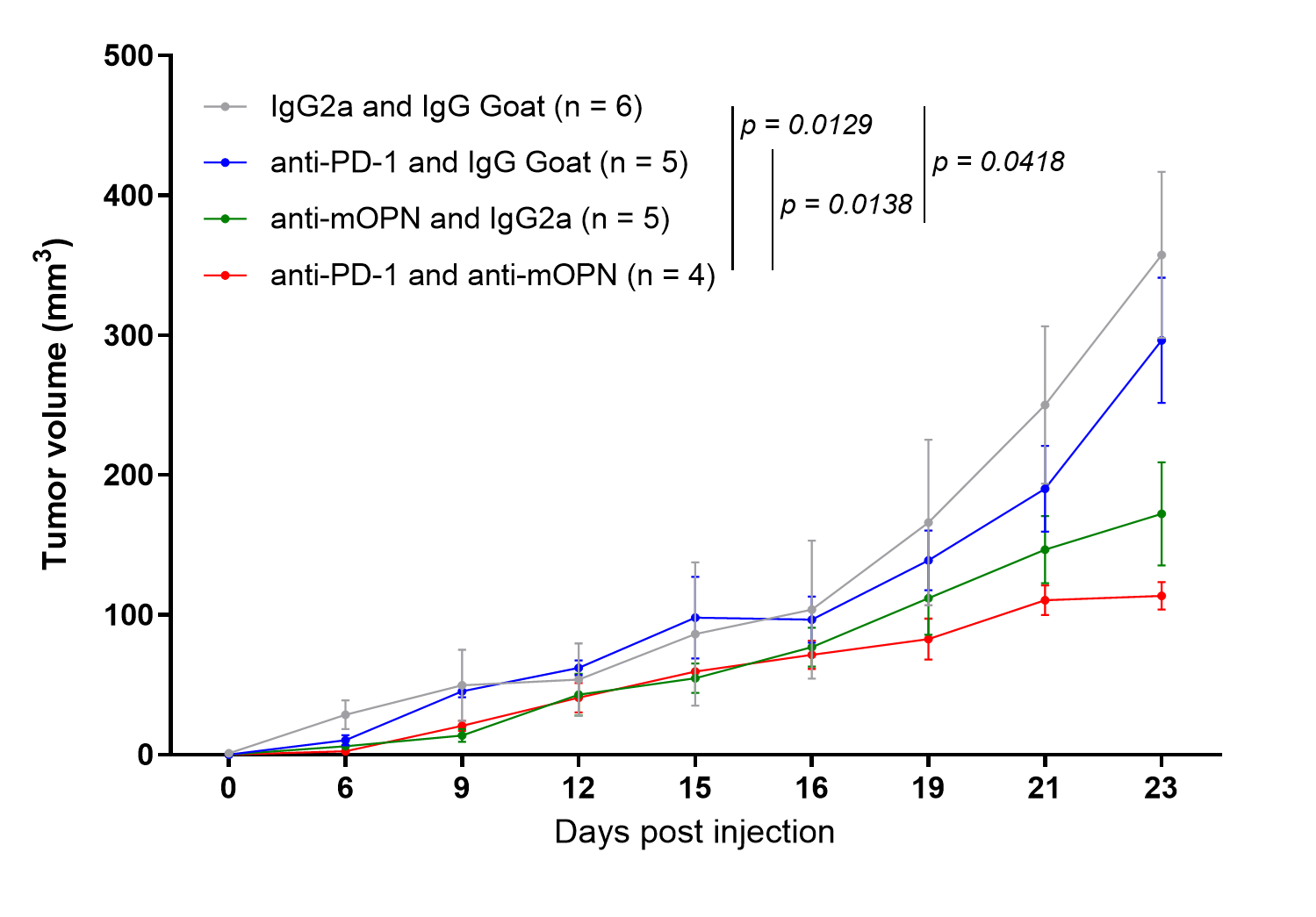
One of the most exciting findings of our study is the potential of targeting osteopontin. In breast tumor-bearing mice, inhibiting osteopontin effectively reduced primary tumor burden and lung metastasis. To our surprise, the former was in tandem with T cell infiltration and activation. Therefore, targeting osteopontin directly elicits an immune-mediated tumor clearance. The relief of T cell exclusion and subsequent activation are especially important in screening and in improving immunotherapies like anti-PD1. Indeed, in an anti-PD1-resistant breast cancer model, we further show that supplementing anti-osteopontin drug yields an additive effect in reducing tumor burden.
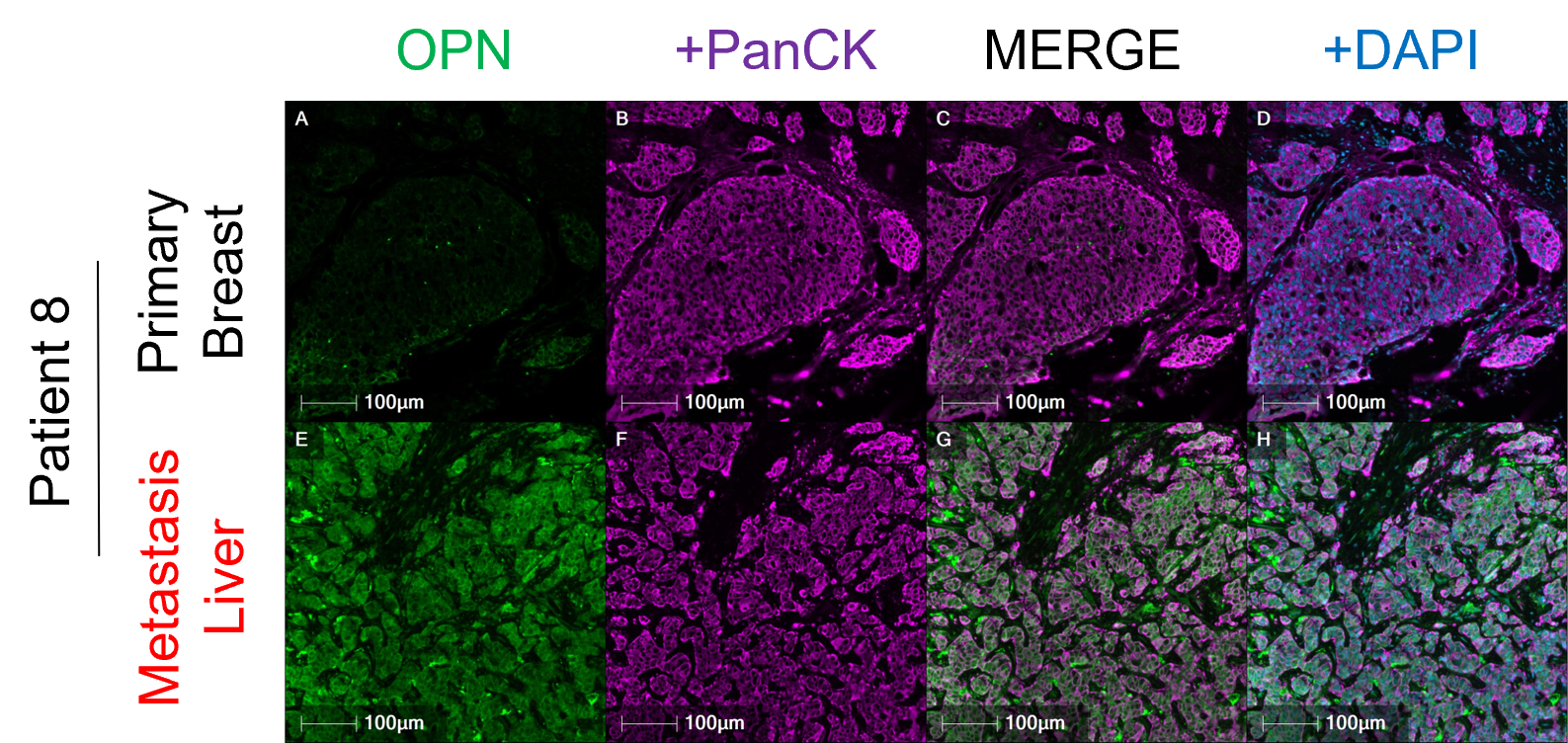
The Bigger Picture: Clinical relevance
Beyond our in vivo and in vitro investigations, human meta-data and patient sample analyses show that osteopontin levels are significantly higher in tumors versus healthy tissues and significantly higher in recurrent metastatic tumors versus patient-matched primary breast tumors. We also found that higher levels of osteopontin in breast tumors are associated with worse prognosis and long-term recurrence, granting it valuable biomarker potential as a predictor of relapse. Beyond breast cancer, osteopontin levels are much higher in many other solid cancers compared to healthy tissue, including cervical, colon, esophageal, head and neck, liver, lung, skin, and stomach cancers.
The Collective Challenge: From remission to cure
The term “remission” holds as much hope as uncertainty. At every stage of cancer progression and treatment, there is a window of opportunity to improve the long-term outcomes for patients. By understanding the mechanisms and the kinetics of these mechanisms as tumors initiate, progress, regress, and recur, we can unveil new targets like osteopontin and therapeutically leverage their global effects to prevent cancer relapse.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in