Parasagittal dura is a potential neuroimmune interface
Published in Neuroscience

Our understanding of cerebrospinal fluid (CSF) flow dynamics and efflux is limited, probably because of difficulties to assess the slow flow and distribution of substances under normal physiological conditions. So far, study objects have for the most part been animals, mainly rodents. Lately, some groundbreaking animal studies have shown how substances in CSF can exchange with the brain and drain to meningeal lymphatic vessels. Based on these studies, our research group has examined patients with a CSF tracer substance and repeated magnetic resonance imaging (MRI) to investigate the distribution of a substance in CSF, the brain and a particular part of the meningeal tissue; the parasagittal dura (PSD). This paper focused on PSD.
PSD: The parasagittal dura
PSD is the meningeal tissue adjacent to the superior sagittal sinus above the vertex of the brain (fig 1) and contains lymph vessels, fluid vacuoles, stroma, blood vessels, arachnoid granulations and immune cells. Historically, arachnoid granulations in PSD have been considered a major CSF efflux route, but the evidence for efflux through the arachnoid granulations under normal physiological conditions is weak. Nevertheless, PSD has functions directly connected with the CSF: 1) Substances from CSF enter PSD and are cleared to lymphatic vessels; 2) PSD acts as a neuroimmune interface where antigen-presenting cells are located and activated by antigens from the CSF. To acquire more knowledge about this little investigated tissue, we segmented PSD on Fluid-attenuated inversion recovery (FLAIR) MRI with a manual method assisted by an artificial intelligence model. PSD showed strikingly large variations in volume between patients (fig 1). To assess these differences, clinical and physiological variables including tracer dynamics in the CSF, PSD and blood, age, sex, sleep quality, intracranial pressure and different intracranial volumes were analysed.
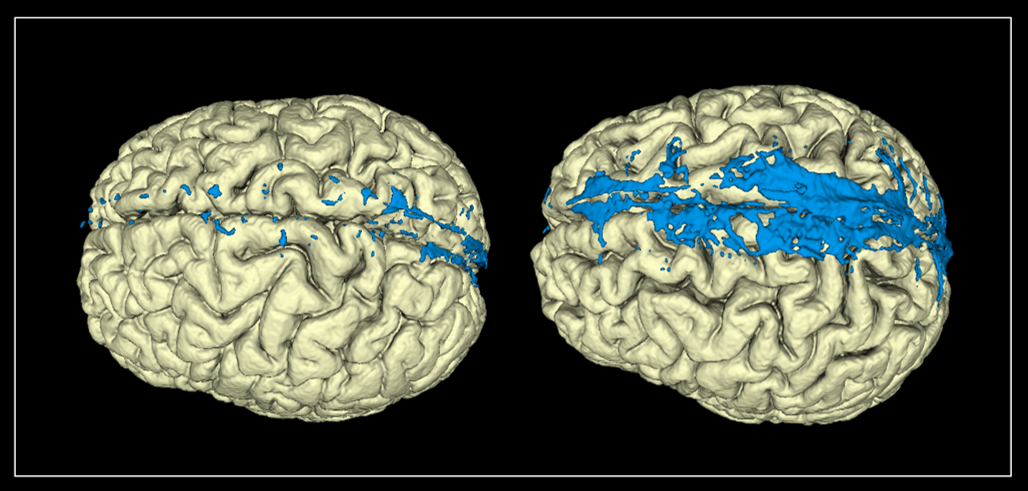
Tracer and imaging
We injected a small amount of gadobutrol, a MRI contrast agent, at the lumbar level followed by MRI scans and blood samples for up to 48 hours; Gadobutrol served as a CSF tracer substance visualised by MRI. Gadobutrol has no known active transporters and does not leak directly into the blood. As so, the tracer follows passively the CSF distribution for a small hydrophilic molecule within the brain tissue, at the brain surface and into the meninges surrounding the brain. The images and the blood samples inform us where and when the tracer is distributed within and outside of the CSF, including PSD. We correlated the tracer enrichment in PSD to PSD volume and found no association. That is, PSD volume is no good marker for CSF clearance via PSD.
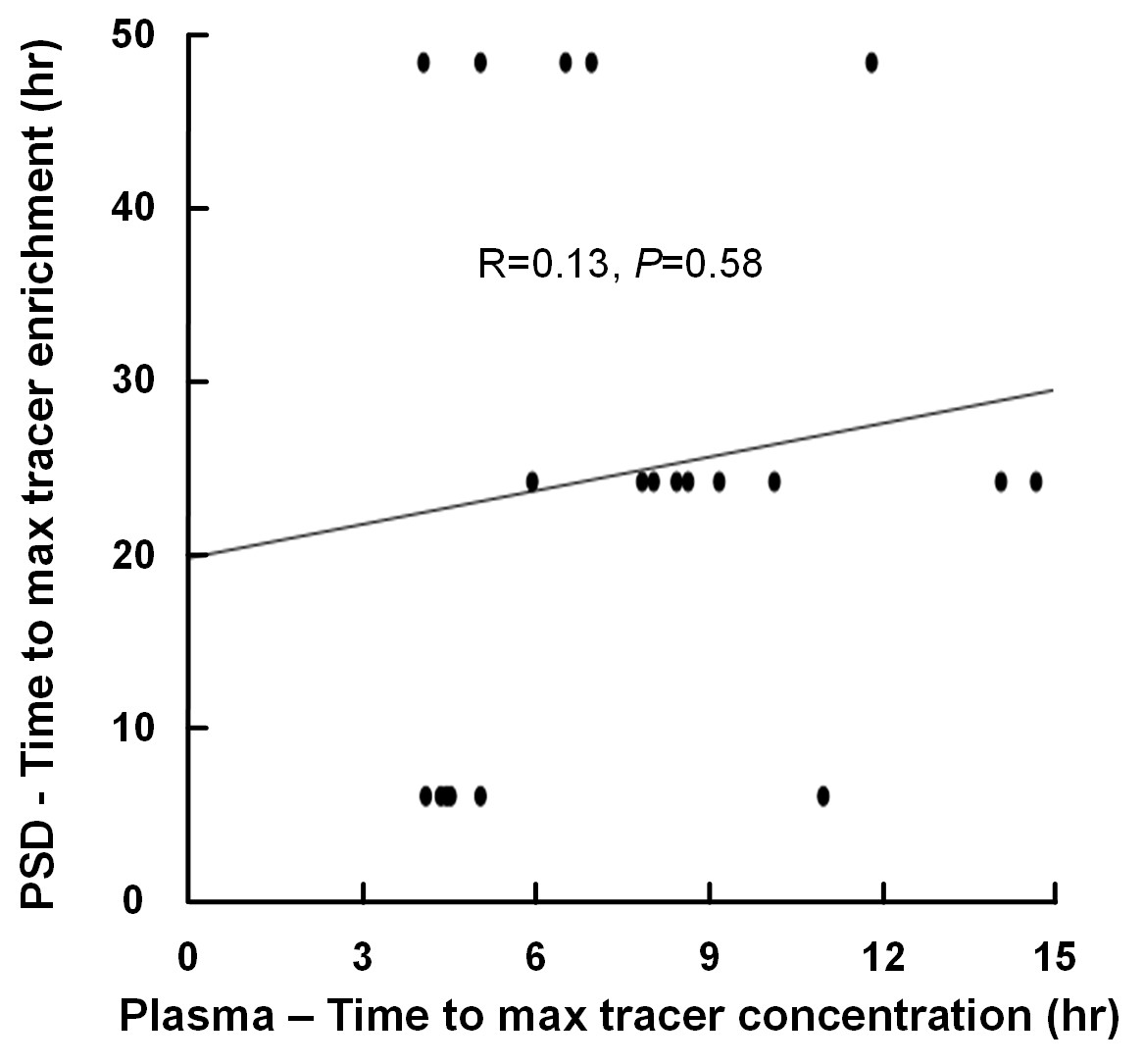
The time to maximum concentration of tracer in the blood occurs far earlier compared to maximum enrichment of tracer in PSD (fig 2). Most tracer has obviously left CSF through other routes than PSD. In the 1960s, De Chiro published tracer studies of CSF dynamics where he observed tracer accumulation at the vertex of the skull after 24 hours. This was described as a result of flow towards a possible efflux route via arachnoid granulations at the vertex of the head. Later, this conclusion has been questioned and our data with both tracer measurements in CSF, PSD and blood and imaging where we observe no bulk flow support the idea that the tracer in the CSF spaces above the brain after 24 hours is merely a result of slow distribution to this space. For safety reasons we inject the tracer at the lumbar level; An intracranial injection would lead to a different temporal correlation between tracer distribution in PSD and blood.
Sleep Quality and intracranial pressure
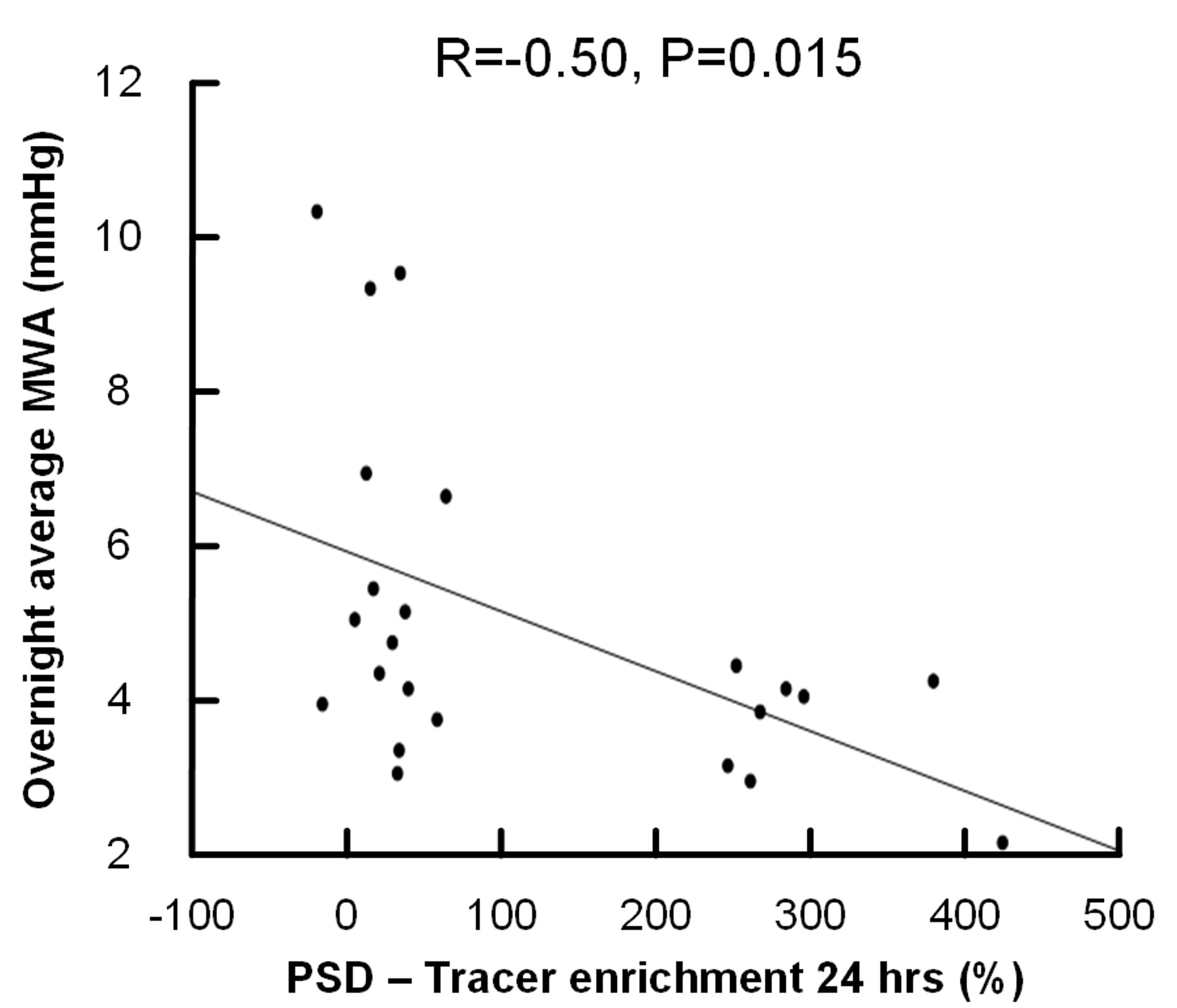
Clearance from the brain differs in wake and sleep state, but we observed no correlation between sleep quality and PSD volume. We showed that pulsatile intracranial pressure (ICP) is associated with lower tracer enrichment in PSD after 24 hours (fig 3) and a smaller PSD volume; We believe the differences in tracer enrichment and volume are caused by altered flow dynamics in CSF and pressure-related volume changes of PSD in patients with increased pulsatile ICP.
Conclusion
Historically, CSF was thought to be resorbed through arachnoid granulations in the meninges at the upper brain convexities. In this work, we find no important role of CSF efflux in PSD, which contains these granulations. Large inter-individual variations in PSD volumes remain poorly explained by tracer dynamics or other assessed variables. These observations support recent animal studies suggesting that the brain has pushed its immunological borders towards the periphery and that PSD is one such interface between the brain and the immune system, with CSF as a carrier of substances in between.
Follow the Topic
-
Communications Biology

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the biological sciences, representing significant advances and bringing new biological insight to a specialized area of research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Signalling Pathways of Innate Immunity
Publishing Model: Hybrid
Deadline: Feb 28, 2026
Forces in Cell Biology
Publishing Model: Open Access
Deadline: Apr 30, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in