Particle contaminants as potential disruptors of biomolecular condensates
Published in Earth & Environment and Cell & Molecular Biology
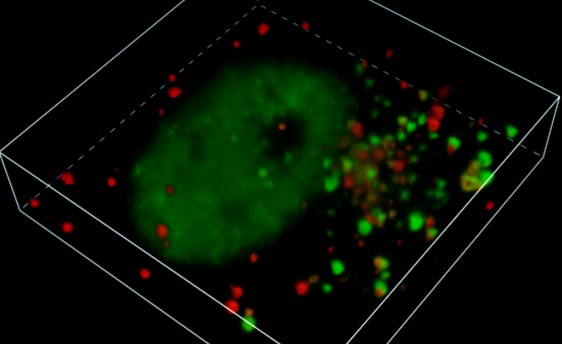
Plastic products have been broadly used nowadays, which ensuing plastic pollution has become a global problem. It is estimated that approximately 11 billion tons of plastic waste will be placed in the environment by 20251. Despite the convenience and economic benefits that plastics bring, their ecological impact cannot be ignored. With the advancement of science and technology and a growing understanding of the plastic pollution problem, people are gradually aware of the potential harm that plastic particles can cause to the environment and organisms. Plastics may undergo continuous fragmentation and shred into micro- or even nano-meter scale particles (termed microplastics and nanoplastics, MNPs) often detected in water, soil, and air. Specifically, airborne MNPs can float and transport in the atmosphere; previous studies indicated that plastic fibers were found in human lung tissue, suggesting that airborne MNPs are inhalable2. Similar to other small particles, MNPs, upon their uptake, can reach the brain and exert a wide range of neurotoxic effects; however, there is still urgent demand to further illuminate the neurotoxic mechanism(s) of MNPs. Mounting evidence indicated that neurodegenerative diseases were highly associated with ambient air pollutants and ultrafine particle exposure3. Recent epidemiological studies have progressively linked airborne particle exposure to the incidence of amyotrophic lateral sclerosis (ALS)4.
In response to cellular stress, physiological TAR DNA-binding protein 43 (TDP-43, a typical ALS-associated pathogenic protein) is driven by liquid-liquid phase separation (LLPS), a process of biomolecules from the dilute aqueous phase to the condensed demixed phase, to form biomolecular condensates and perform its physiological functions. Notably, aberrant phase separation is gradually explaining the molecular mechanisms of the ambiguous neurodegenerative disease-associated protein aggregation5. Given the unique properties of nanoparticles, it is reasonable to assume that internalized nanoplastics may have contact with TDP-43 condensates formed by phase-separation-driven formation. We are curious about if/how particle contaminants affect TDP-43 phase separation and what intervention is needed in neurodegenerative disease progression. Furthermore, multiple nanoparticles have been reported to promote pathological trends in neurodegenerative disease-associated proteins6, 7, which encourages us to explore the relationship between nanoplastics and TDP-43 phase separation further.
While our laboratory has been focused on studying the molecular mechanisms underlying the disease development induced by particle contaminants and the interactions between nanoparticles and protein coronas for several years, we have not been studying biomolecular condensation in that context. The interaction between nanoparticles and proteins contributes to the formation of protein crowns, which is a protein enrichment process dominated by the properties of the nanoparticles. Meanwhile, biological phase separation drives protein enrichment through multivalent weak interactions between molecules. They possess similar enrichment ability for biological macromolecules. In addition, nanoparticles can affect the residues of proteins due to the interfacial forces between the two that may affect each other’s surface groups. Based on the importance of residues for phase separation, we preliminarily hypothesized that the nanoplastics adsorbed or contacted TDP-43, which leads to compromised TDP-43 phase separation.
Due to the unique properties of nanoplastics, it may become a persistent exogenous stressor for the cell; therefore, protein homeostasis and quality control are challenged within the cell. When exposed to polystyrene nanoplastics (PS), cells endure internal oxidative stress, which leads to the aggregation of TDP-43, triggering ALS-like characteristics. Additionally, the oxidized heat shock protein 70 (Hsp70) fails to escort TDP-43 back to the nucleus. We found that the cytoplasmic accumulation of TDP-43 facilitates the formation of a complex between PS and TDP-43, enhancing TDP-43’s condensation and solidification. These findings are corroborated through in silico and in vivo assays. Meanwhile, several publications linked PS to Parkinson’s disease-associated pathology protein (α-synuclein) at the cellular level6, 8, 9. Altogether, our work illustrates a unique toxicological mechanism induced by nanoparticles. It also sheds new light on the elusive links between environmental pollutants and the grim landscape of neurodegenerative disorders, offering a sobering glimpse into the possible consequences of our modern plastic age. With these revelations, we stand at the threshold of a deeper understanding, where the pursuit of scientific knowledge meets the urgency of environmental stewardship.
More work is required to explore the potential molecular mechanisms of environmental pollutant-mediated biomolecular condensates to enable the development of novel prevention and targeted intervention strategies.
Reference
- Brahney J, Hallerud M, Heim E, Hahnenberger M, Sukumaran S. Plastic rain in protected areas of the United States. Science 2020, 368(6496): 1257-1260.
- Pauly JL, Stegmeier SJ, Allaart HA, Cheney RT, Zhang PJ, Mayer AG, et al. Inhaled cellulosic and plastic fibers found in human lung tissue. Cancer Epidemiol Biomarkers Prev 1998, 7(5): 419-428.
- Chen H, Kwong JC, Copes R, Tu K, Villeneuve PJ, van Donkelaar A, et al. Living near major roads and the incidence of dementia, Parkinson's disease, and multiple sclerosis: a population-based cohort study. Lancet 2017, 389(10070): 718-726.
- Yu Z, Peters S, van Boxmeer L, Downward GS, Hoek G, Kioumourtzoglou MA, et al. Long-Term Exposure to Ultrafine Particles and Particulate Matter Constituents and the Risk of Amyotrophic Lateral Sclerosis. Environ Health Perspect 2021, 129(9): 97702.
- Wang B, Zhang L, Dai T, Qin Z, Lu H, Zhang L, et al. Liquid-liquid phase separation in human health and diseases. Signal Transduct Target Ther 2021, 6(1): 290.
- Liu Z, Sokratian A, Duda AM, Xu E, Stanhope C, Fu A, et al. Anionic nanoplastic contaminants promote Parkinson's disease-associated alpha-synuclein aggregation. Sci Adv 2023, 9(46): eadi8716.
- Mohammad-Beigi H, Zanganeh M, Scavenius C, Eskandari H, Farzadfard A, Shojaosadati SA, et al. A Protein Corona Modulates Interactions of alpha-Synuclein with Nanoparticles and Alters the Rates of the Microscopic Steps of Amyloid Formation. ACS Nano 2022, 16(1): 1102-1118.
- Jeong A, Park SJ, Lee EJ, Kim KW. Nanoplastics exacerbate Parkinson's disease symptoms in C. elegans and human cells. J Hazard Mater 2024, 465: 133289.
- Liang X, Andrikopoulos N, Tang H, Wang Y, Ding F, Ke PC. Nanoplastic Stimulates the Amyloidogenesis of Parkinson's Alpha-Synuclein NACore. Small 2024, 20(14): e2308753.
Follow the Topic
Your space to connect: The Myeloid cell function and dysfunction Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine and Cell Biology!
Continue reading announcement


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in