Petrological evidence for deep subduction of organic carbon to subarc depths
Published in Earth & Environment

How it started
Oceanic sediments constitute a substantial carbon source within subduction zones, encompassing both inorganic and organic carbon components that are transported into the subduction factory. Understanding the fate of these carbon inputs in subduction zones is crucial for gaining insights into the global carbon cycling throughout Earth's history. While the significance of carbonate in the subduction-zone carbon cycle has been extensively investigated in recent decades, the importance of organic carbon within subduction-zone lithologies and deep fluids has been relatively overlooked. The deep subduction of reduced organic carbon has impacts on various geological events, including the Great Oxygenation Event, the Lomagundi event, arc carbon isotopic signature and the formation of deep diamonds with light carbon isotopic features.
Petrologist Prof. Zhang Lifei from Peking University, China, has long been working on metamorphic geology in the southwest Tianshan orogenic belt. Prof. Zhang and his students have identified coesite, an ultrahigh-pressure (UHP) index mineral, in various rocks such as eclogites, metapelites, marbles and Ti-chondrodite- and Ti-clinohumite-bearing serpentinites in the southwest Tianshan. Approximately twenty years ago, Prof. Zhang investigated the UHP metamorphism of carbonates in pelitic schists from the Tianshan region and suggested that these carbonates were subducted to subarc depths. In recent years, Prof. Zhang and his students’ interests have been piqued by graphite, an undervalued yet essential accessory mineral in pelitic schist, leading them to explore the fate of organic carbon hosted in oceanic crust sediments during subduction and whether graphite produced through graphitization of organic carbon can also reach subarc depths.
The problem
Diamond, a high-pressure polyphase of graphite, is estimated to be formed in the lithospheric mantle at depths exceeding 140 km or under pressures greater than 4.5 GPa. An intriguing debate related to crustal recycling into the deep mantle regards the involvement of subduction-derived C in the genesis of diamond. According to the literatures, some diamonds exhibit lighter carbon isotope characteristics (i.e., lower than mantle range), particularly ophiolitic diamonds, which show δ13C ranging from −29‰ to −18‰ with a peak value of −25‰. Various theories have been posited to explain the light δ13C compositions of these diamonds, including the primordial carbon reservoir, intra-mantle isotopic fractionation, or the incorporation of subducted biogenic/organic carbon32. However, mantle Sr, Nd, and O isotopic geochemistry data rule out the preservation of primordial carbon isotope heterogeneity in Earth’s mantle. Moreover, the narrow range of δ13C values from individual diamonds does not support isotope fractionation from a normal mantle carbon source with δ13C of –5 ± 2‰. Therefore, the feature of light carbon recorded by ophiolitic diamonds possibly reflects the composition of subducted organic carbon. However, the lack of robust petrological evidence makes this hypothesis arguable.
The solution and results
We conducted fieldwork in the Tianshan UHP area where coesite has been reported from the pelitic schists and collected graphite-bearing pelitic schists. By preliminary microscopic observation, we found many radial cracks developed in the garnet of the graphite-bearing pelitic schists, with quartz crystals at the center of the cracks. This structure, which we termed the ‘coesite pseudomorph’, was caused by the conversion of formerly present coesite to quartz during decompression. We then searched for transparent inclusions in the graphite-bearing garnet crystals and finally identified the coesite inclusions within garnet (Figure 1) by Raman spectroscopy.
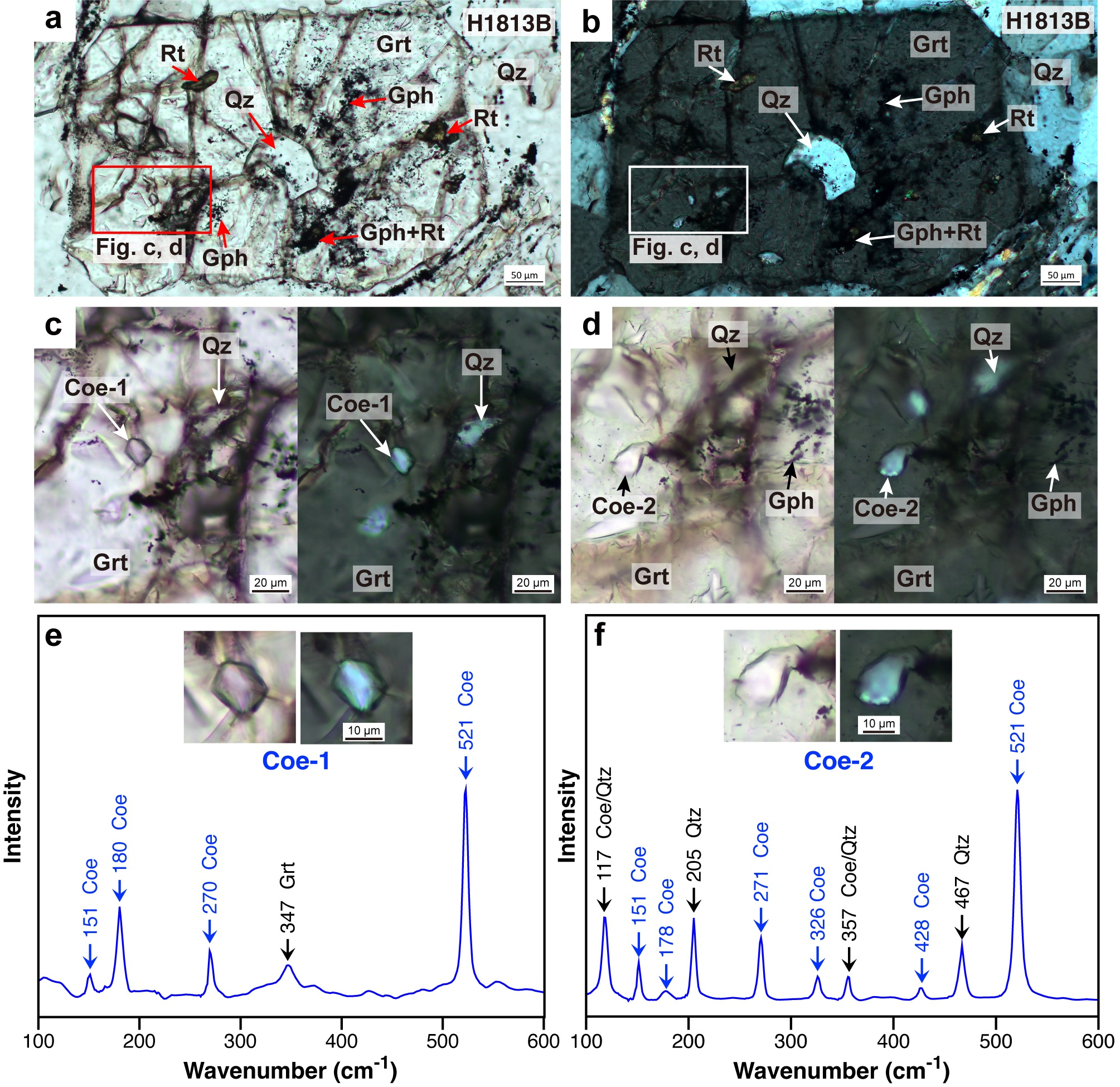
Figure 1. Coesite and graphite inclusions in garnet.
a and b Two coesite (Coe) crystals were found in the garnet (Grt), which contains quartz (Qz) with radial fractures. Graphite (Gph) and rutile (Rt) are contained in garnet. c and d Coesite inclusions in garnet. e and f Raman spectra of coesite in garnet c and d.
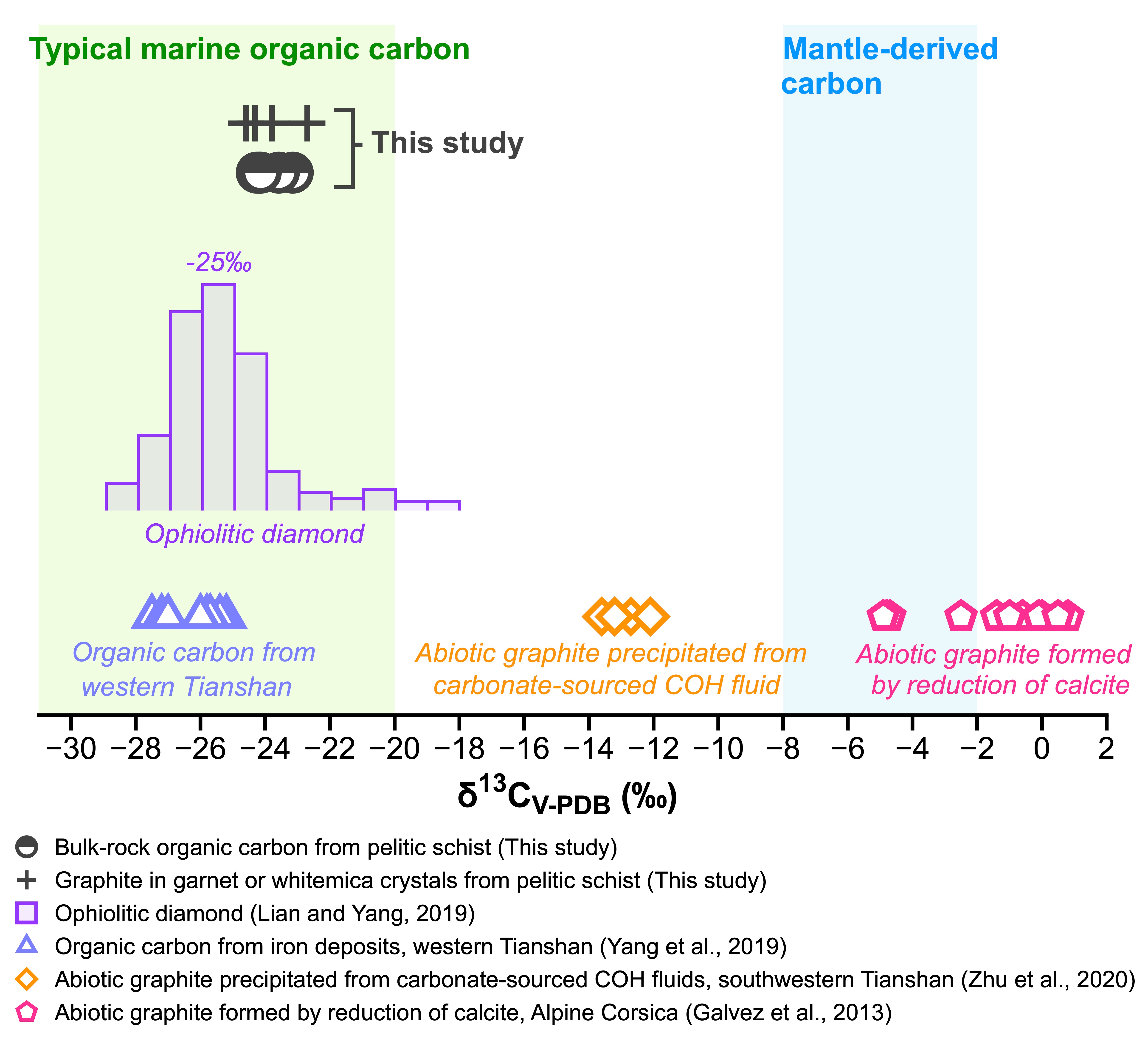
Figure 2. δ13C values of bulk rock organic carbon and graphite contained in garnet and whitemica crystals from the coesite- and graphite-bearing pelitic schists in the Chinese southwestern Tianshan UHP belt.
Next, we performed organic carbon isotope analysis on graphite-bearing pelitic schists. For each sample, we prepared three types of powders: (i) whole-rock powder, (ii) picked graphite-bearing garnet powder, and (iii) picked graphite-bearing white mica powder. These pelitic schists (bulk-rock powder) have homogeneous δ13CTOC values of −24.7 to −22.5‰ (Figure 2), which fall in the range of typical marine organic matter. The δ13CTOC of picked graphite-bearing garnet crystals and picked graphite-bearing whitemica (phengite and paragonite) are in agreement with that of the bulk rock (Figure 2). This consistency suggests that graphite serves as the primary organic carbon phase in these samples. Our study thus provides petrologic evidence that organic carbon in surface oceanic crustal sediments can be subducted to subarc depths.
Future directions and implications
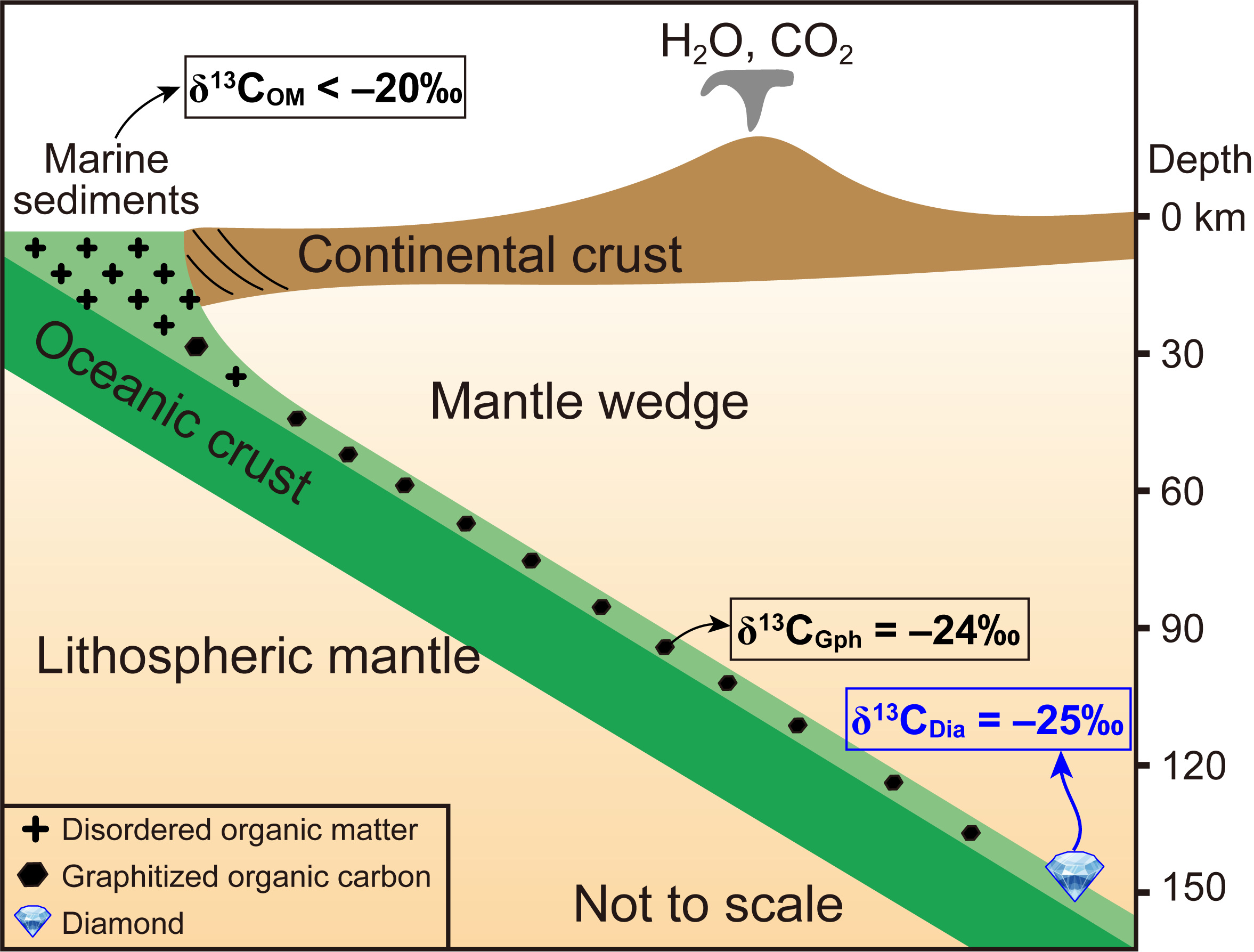
Figure 3. Cartoon illustrating the fate of organic matter in subduction zones.
Disordered organic matter (OM) (δ13COM < −20‰) contained in marine sediments undergoes partial to complete graphitization with increasing temperature in subduction zones. The results presented in this study suggest that these disordered organic matter from marine sediments subducted to subarc depths (around 90 km, coesite-stable field), forming graphite with δ13CGph of −24‰. This subduction of surface organic carbon may contribute to the formation of ophiolitic diamonds with light δ13C values (peak value of −25‰).
With the increasing subduction depth, surface-derived disordered organic carbon is transformed to crystalized graphite at subarc depth (around 90 km) and subsequently to diamonds at lithospheric mantle depth (140 km), as illustrated in Figure 3. Further studies are needed to fully elucidate the mechanisms and processes involved in the transition from surface organic carbon to graphite and then to diamonds.
Follow the Topic
-
Communications Earth & Environment

An open access journal from Nature Portfolio that publishes high-quality research, reviews and commentary in the Earth, environmental and planetary sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Archaeology & Environment
Publishing Model: Hybrid
Deadline: Mar 31, 2026
Drought
Publishing Model: Hybrid
Deadline: Mar 31, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in