Phenotyping ageing
Published in Bioengineering & Biotechnology

As a naïve, bright-eyed chemical engineering student at the City College of New York, I quickly learned that to have a meaningful impact as an engineer requires creativity, a strong base in scientific fundamentals and an eagerness to collaborate. Growing up on the tiny Caribbean island of Grenada, a country with limited resources and few trained engineers, fostered with in me the humility to learn from others and the tenacity to push forward. Being classically trained in chemical engineering, my exposure to biology was limited, and at times dampening my ability to see the direct translational links between engineering and medicine. Joining Johns Hopkins’ Chemical and Biomolecular engineering program, I was particularly excited, not only because of the cutting edge research taking place within the department, but also because of the opportunity to closely collaborate and learn from world-class clinicians and researchers. I was fortunate to join the lab of Dr. Denis Wirtz, then the co-director of the University’s Institute for Nanobiotechnology (INBT). He sported a strong track record of technology development in a highly collaborative environment. Early on during my PhD, I collaborated with a team of pathologists and engineers to develop a high-throughput cell-phenotyping platform, which we then used to identify a signature of pancreatic cancer metastasis. Little did I know at the time, that I would find myself collaborating with yet another team of forward-thinking clinicians to use this technology to study ageing.
I remember my first encounter with Dr. Jeremy Walston, a practicing Geriatrician and the Principal Investigator of the Johns Hopkins Older Americans Independence Center. It was clear from that meeting that he was very knowledgeable and passionate about ageing. The questions that he posed appealed to the engineer within me; however, it was not initially clear how to go about answering them. This research was built around the concept of frailty in older individuals, which Dr. Walston and his team had been studying for years. The core questions stemmed from clinical observations made during routine patient visits. For instance, why is it that three 75 year olds could have dramatically different health spans and life expectancies? What are the biological reasons that enable one 75-year-old to comfortably run a marathon (robust), while another can barely stand on their own from a seated position (frailty), and the other functions somewhere in between (intermediate)? Chronic diseases can certainly impact function and health. Our team was eager to identify ageing-related biological changes that may influence the differences in the functional health of older adults. I believe that it was this nucleation of Phillip, Wirtz and Walston that formed the critical mass needed for the development of the technology and vision described now in Nature Biomedical Engineering.
Our hypothesis was that deficiencies at the pathophysiological level likely stemmed from functional impairments in the cells themselves. I applied our robust cell-based technology to help identify age-based differences in cellular function. The long-term goal was to identify ageing-related changes that could be modified and monitored to improve healthspan, and ultimately longevity. Data derived from cells of ‘apparently healthy’ individuals spanning 10 decades were fed into a mathematical model. We found that a multivariate approach resulted in a clear age-dependent stratification of individuals.
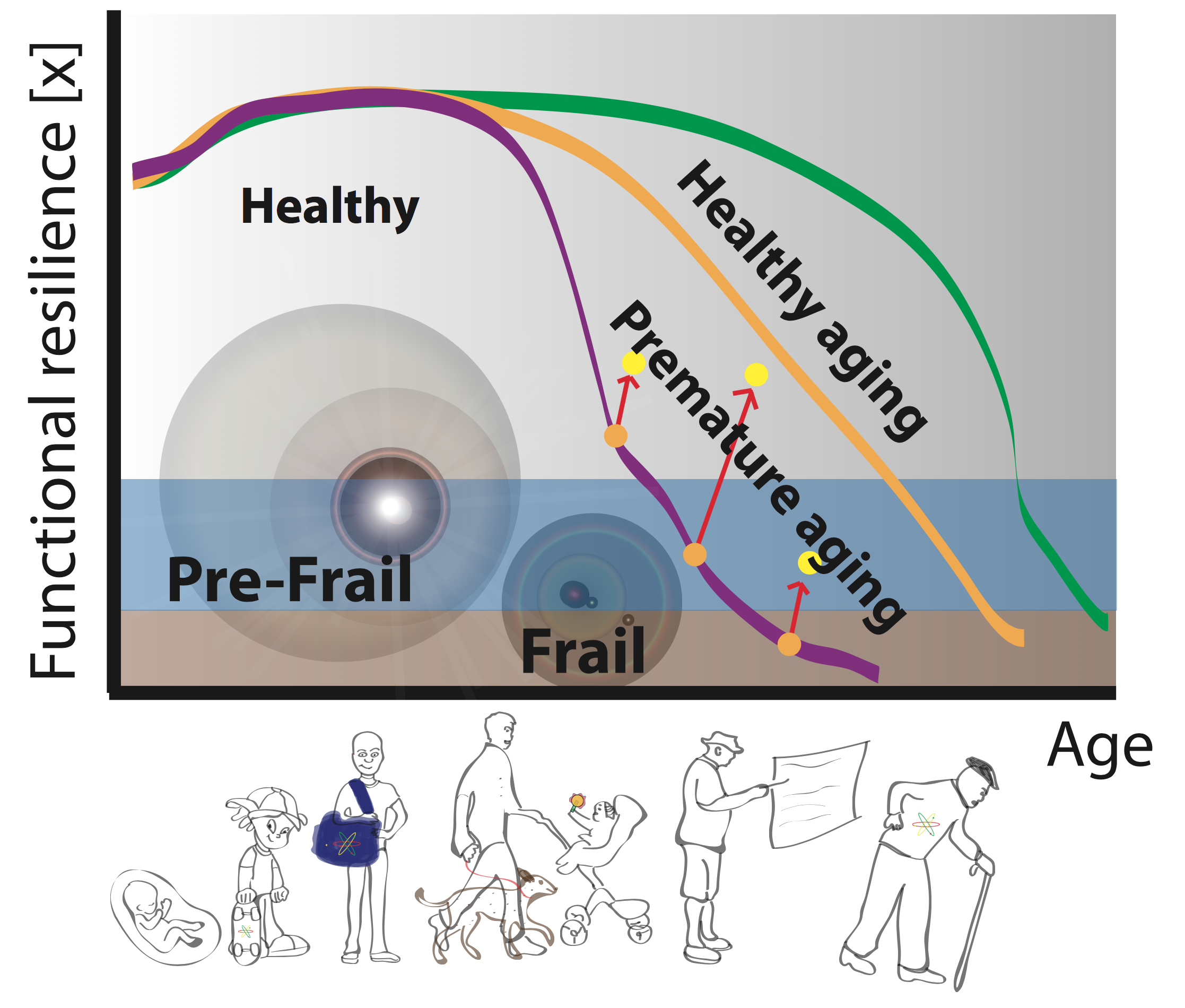
Figure 1. Phase diagram of ageing. Ageing trajectories for premature/accelerated (purple), healthy (orange) and
slowed (green), with delineations of pre-frail and frail regimes. Arrows illustrate the potential for clinical-course
correction on the basis of monitoring and interventions based on cellular-age prediction.
Our findings, although robust and encouraging, are just the beginning in terms of potential endpoint applications of this technology, and possibly go beyond ageing. It could provide a window into a person’s health at the cellular level in any age group. With ongoing studies, we hope to expand other translational avenues, including studies for toxicology screening of topical biotherapeutics, and enhanced skin-graft matching based on phenotype-mapping of cellular properties.
Working in a highly collaborative environment with creative mentors who have seemingly limitless curiosities has not only fostered the development of this research, but also shaped the bioengineer that I am today.
Our paper: Phillip, J. M. et al. Biophysical and biomolecular determination of cellular age in humans. Nat. Biomed. Eng. 1, 0093 (2017).





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in