Photoacoustic vector tomography for deep haemodynamic imaging
Published in Healthcare & Nursing, Bioengineering & Biotechnology, and Protocols & Methods
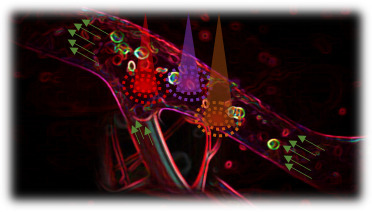
Non-invasive imaging of deep blood vessels for mapping haemodynamics remains an open quest. Pure optical imaging techniques, such as Doppler-based methods, speckle-based methods, and red blood cell tracking methods have been reported to map the dynamics of blood flow due to their intrinsic optical contrast of blood. However, these techniques suffer from shallow depth penetration within the optical diffusion limit (< 1 mm1) due to strong ballistic light attenuation in biological tissue.
Conversely, photoacoustic computed tomography (PACT) combines optical absorption contrast with low scattering ultrasonic detection to enable high spatiotemporal resolution and deep tissue imaging of endogenous chromophores, such as haemoglobin. PACT uses wide-field illumination coupled with an array of ultrasonic detectors to image vasculature at depths beyond the optical diffusion limit with acoustic resolution2. However, to date, measuring blood flow in deep tissue with PACT has been challenging due to 1) the inability to resolve individual red blood cells (RBCs); 2) the photoacoustic signals within the lumen region of a vessel are suppressed relative to the signals at the boundaries due to the random summation of absorption signals from millions of RBCs in each lumen imaging voxel3.
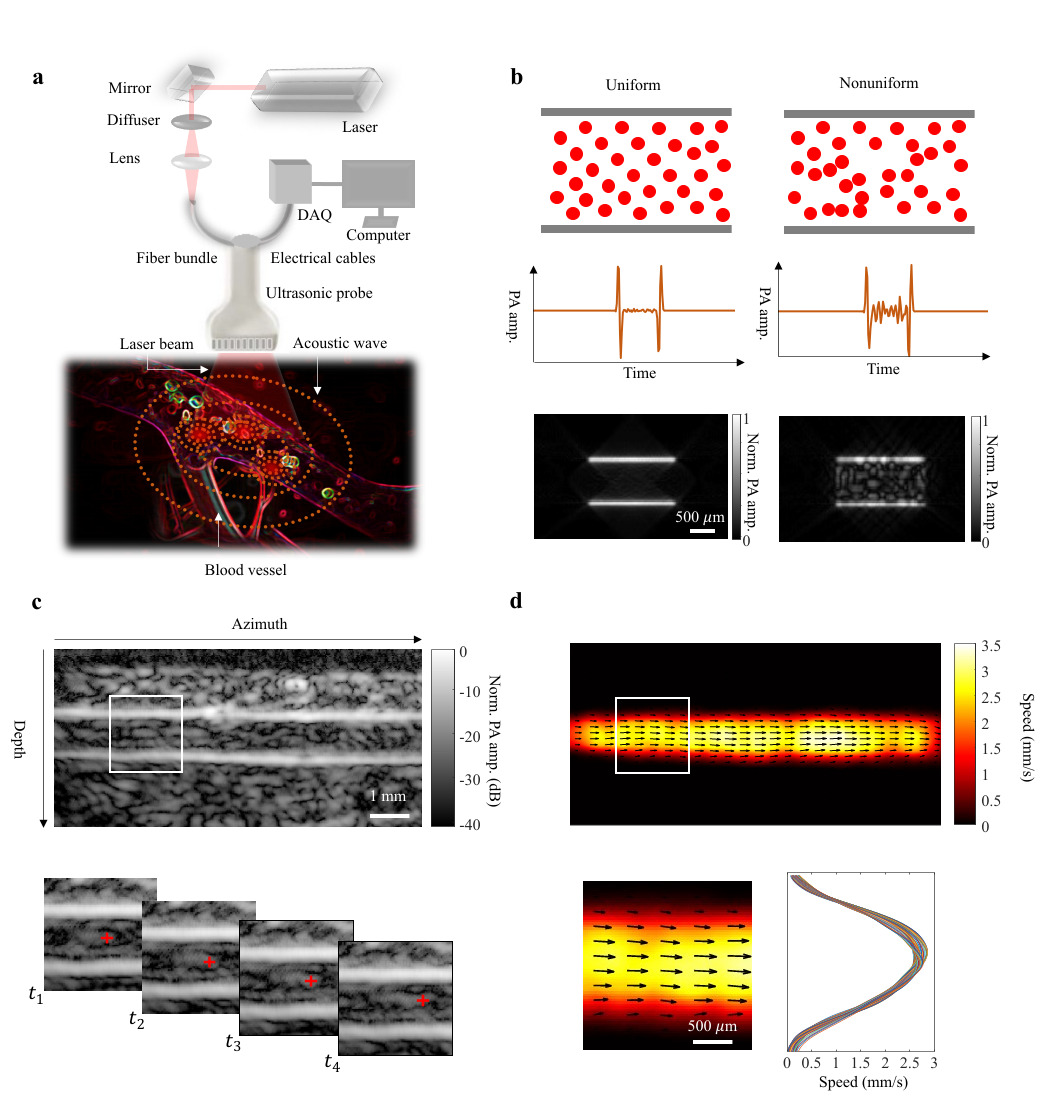
Herein, we present photoacoustic vector tomography (PAVT) as a framework to achieve the first known vector maps of human blood flow by photoacoustics beyond the optical diffusion limit. The key features of this framework (with further details provided in Fig. 1) can be summarized as follows: 1) The synergy between the spatial heterogeneity of blood and the photoacoustic contrast produces strong photoacoustic signals in the lumen of the blood vessels. 2) Successive compilation of single-shot, wide-field photoacoustic images allows direct visualization of the blood flow throughout the lumen. 3) Applying pixel-wise motion estimation algorithms to the reconstructed images generates blood flow vector maps with speed and direction quantification. Through simulation and phantom validation, we demonstrated that PAVT blood flow measurement is facilitated by the heterogeneity of the blood, and we demonstrated in vivo that vector flow maps could be obtained in blood vessels greater than 5 mm in depth. This work thus establishes PAVT as a viable imaging technique for monitoring and diagnosing vascular diseases and mapping circulatory system function.
In its current stage, PAVT may be capable of clinical implementation, as it extends the photoacoustic blood flow measurement to five times beyond the optical diffusion limit. In principle, PAVT is not limited to the linear array probe that we employed in this work, and future work may therefore extend this technique to other array geometries such as the ring array and hemispherical array that provide more complete acoustic views for enhanced image quality. Future studies may also extend this approach to other regions of the human body for applications such as breast cancer and functional brain imaging.
PAVT outperforms existing pure optical methods for deep haemodynamic imaging and complements ultrasound imaging by providing both haemoglobin-based molecular contrast. The clinical impact of PAVT will be fully realized when we can perform metabolic imaging of physiological systems. Although methods such as Doppler ultrasound and pulse oximetry can each individually image blood flow and sense systemic oxygen saturation without imaging, respectively, PAVT can simultaneously image these physiological parameters, both of which are required to characterize the metabolic rate of oxygen consumption. As such, PAVT may eventually provide simultaneous measurements of cerebral blood flow, haemoglobin concentration, and oxygen saturation for functional human brain imaging, in addition to aiding in the early detection of hallmarks of cancer, such as angiogenesis and hypermetabolism.
References
1. Wang, L. V. & Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 335, 1458–1462 (2012).
2. Yao, J. & Wang, L. V. Photoacoustic brain imaging: from microscopic to macroscopic scales. Neurophotonics 1, 011003 (2014).
3.Guo, Z., Li, L. & Wang, L. V. On the speckle-free nature of photoacoustic tomography. Med. Phys. 36, 4084–4088 (2009).
These results were recently published in Nature Biomedical Engineering: Y. Zhang, J. Olick-Gibson, A. Khadria, and L. V. Wang*, Photoacoustic vector tomography for deep haemodynamic imaging, Nature Biomedical Engineering, 2023, 10.1038/s41551-023-01148-5.
Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in