Pillararene incorporated metal−organic frameworks for supramolecular recognition and selective separation
Published in Chemistry

In Prof. Feihe Huang’s research group at Zhejiang University, we focus on discovering supramolecular chemistry for functionalization and applications. We initiated collaborative projects with Prof. Jonathan L. Sessler’s and Zhijie Chen’s groups on the interdisciplinary research between supramolecular chemistry and porous materials a few years ago. We intend to introduce precise supramolecular interactions into porous materials such as metal−organic frameworks (MOFs). While working on a project on the pillar[5]arene-incorporated MOFs, we succeeded in determining the precise pillar[5]arene host structure in a MOF crystal, and found this kind of crystalline materials could recognize paraquat and 1,2,4,5-tetracyanobenzene in solution and selectively remove trace pyridine from toluene with relative ease.
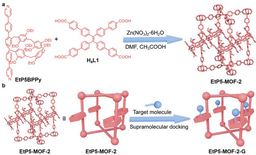
Why pillar[5]arene incorporated MOFs: Crystalline frameworks containing incorporated flexible macrocycle units can afford new opportunities in molecular recognition and selective separation. However, such functionalized frameworks are difficult to prepare and challenging to characterize due to the nature of flexible macrocycles. We reported the design and synthesis of a set of MOFs containing pillar[5]arene units. Single crystal X-ray diffraction analysis revealed an interpenetrated network that appears to hinder the rotation of the pillar[5]arene repeating units in the frameworks, and it therefore results in the successful determination of the precise pillar[5]arene host structure in a MOF crystal. We characterized and identified a pillararene-incorporated MOF structure with atomic resolution. These MOFs can recognize paraquat and 1,2,4,5-tetracyanobenzene in solution and selectively remove trace pyridine from toluene. This work represents a critical step towards the synthesis of macrocycle-incorporated crystalline frameworks with well-defined structures and functional utility.
Motivation and key challenges: In principle, macrocycle-based crystalline framework systems offer several prospective advantages, including: 1) enhanced regulation of the pore structures allowing for the fine-tuning of the molecular recognition and separation capabilities; 2) better accessibility to active recognition sites; 3) efficient diffusion of guest molecules; 4) clearer insights into structure−property relationships since each recognition site is isolated and amenable to independent study. These advantages endow them priority in molecular recognition and separation. Unfortunately, crystalline frameworks incorporating well-defined macrocyclic subunits remain challenging to prepare and difficult to characterize (Chem. Soc. Rev. 2022, 51, 8378). Up to now, the presence of such species was always inferred from indirect measurements and the exact structure was unknown. The present study goes beyond synthesis and structure. For example, although pillararene-based MOFs have been reported (J. Am. Chem. Soc. 2012, 134, 17436), their structural details remain recondite because rotations of the pillararene subunits can lead to disorder within what are presumably overall periodic frameworks.
Key Findings: In our work, we have designed and synthesized a set of MOFs incorporating pillar[5]arene motifs denoted as MeP5-MOF-1, MeP5-MOF-2, MeP5-MOF-3 and MeP5-MOF-4. By comparing the structures of these MOFs determined by SCXRD analysis we conclude that network interpenetration in the case of MeP5-MOF-2 plays a crucial role in allowing the pillar[5]arene units in the frameworks to be resolved while the others possess open networks. We suggest that hindering the rotation of the pillar[5]arene repeating units eliminates their crystallographic disorder within the frameworks. In other words, the “structure” problem of receptor-incorporated MOFs has been solved and the solution was reported in this work. Moreover, both MeP5-MOF-1 and MeP5-MOF-2 were found to adsorb two prototypical guests, paraquat and 1,2,4,5-tetracyanobenzene. In contrast, the pillar[5]arene-free control MOF, named Model-MOF-1, proved relatively ineffective. In addition, both MeP5-MOF-1 and MeP5-MOF-2 could be used to achieve the convenient separation of pyridine from toluene with efficiency. The observed selectivity is rationalized on the basis of single crystal structure analyses.
Impact: Our work shows how a structurally characterized set of pillar[5]arene-containing MOFs may be used to recognize paraquat and 1,2,4,5-tetracyanobenzene in solution and selectively remove trace pyridine from toluene. The associated process is facile and completed readily. These applications represent little more than the tip of the iceberg of what we believe can be achieved using pillararene-based MOFs. We thus expect that the fundamental understanding gained from this research will promote the development of both supramolecular chemistry and crystalline framework materials. Given transformational utility and the associated impact, we expect this work will appeal to the broad readership and be of interest to chemists, materials scientists and students at all levels. In addition, we believe the joined efforts from researchers from different backgrounds will lead to more interdisciplinary research outcome, and other collaborative research data are on the way to be published.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in