Polyoxometalate functionalization by light-dependent coupled reaction equilibria
Published in Chemistry

Here, we report a coupled set of light-dependent and light-independent reaction equilibria which control the mono- or di-metal-functionalization of a prototype molecular vanadium oxide cluster. Comprehensive mechanistic analyses show that coordination of a Mg2+ ion to the species {(NMe2H2)2[VV12O32Cl]}3- results in formation of the mono-functionalized {(NMe2H2)[(MgCl)VV12O32Cl]}3- with simultaneous release of a NMe2H2+ cation. Irradiation of this species with visible light results in one-electron reduction of the cluster shell, exchange of the second NMe2H2+ with Mg2+, and formation / crystallization of the di-metal-functionalized [(MgCl)2VIVVV11O32Cl]4-. Mechanistic studies show how stimuli such as light or competing cations affect the coupled equilibria. Transfer of this synthetic concept to other metal cations is also demonstrated, highlighting the versatility of the approach.
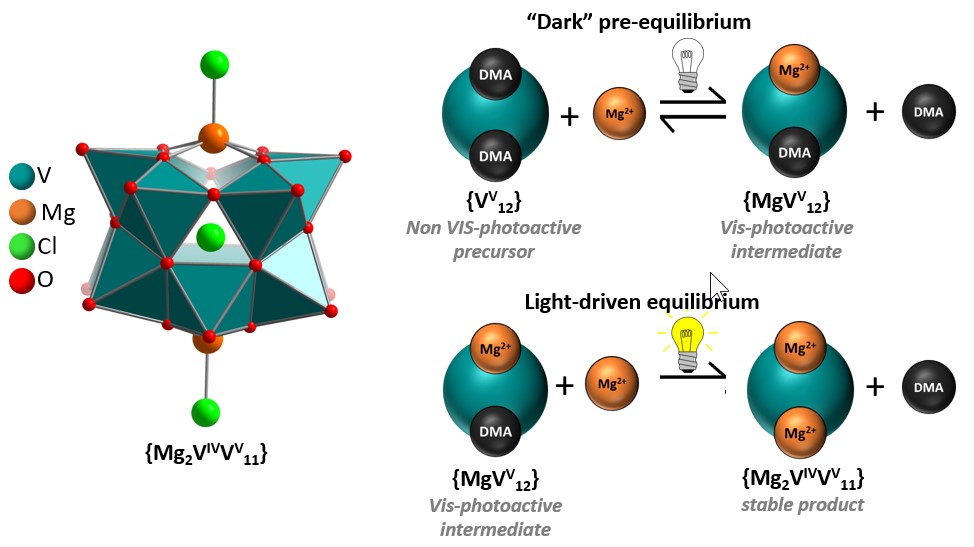
Left: Illustration of the di-Mg-functionalized polyoxovanadate species {Mg2V12}.
Right: Illustration of the light-independent and light-dependent coupled reaction equilibria.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in