Powerhouses on the Edge: Unveiling the Connection between Mitochondrial Dysfunction and Bipolar Disorder
Published in Neuroscience
Bipolar disorder (BD) is a chronic psychiatric disease with a high social and functional burden, affecting about 1% of the world's population. Research into the pathophysiology of BD has remained elusive over the years, perhaps because of a multifactorial etiology that involves genetics, neurochemicals, and the environment. According to genetic studies, BD is more likely to be transmitted maternally, and BD patients tend to have abnormal mitochondrial DNA (mtDNA). Aside from bioenergetic dysfunction, BD patients also have oxidative stress, abnormal mitochondrial morphology, and damaged mtDNA.
There is a new paradigm that mitochondria play a non-energetic role in cellular adaptation to stress, impacting cellular resilience and acting as an allostatic load source for the system. By changing morphology, dynamics, and function, mitochondria lose their capability to recalibrate and keep cell homeostasis, a phenomenon known as mitochondrial allostatic load (MAL). It is also possible that chronic stress, such as recurrent affective episodes, can cause mitochondria to malfunction, raising allostatic load and increasing the risk of immune-cardiometabolic dysregulation and functional and cognitive impairment. Impaired mitochondrial quality control due to deficient trafficking/removal of dysfunctional mitochondria and defective mitochondrial biogenesis indicates that mitochondria can no longer maintain homeostasis, contributing to allostatic load and apoptosis activation. Activation of cell death can result in the release of mtDNA to the circulation (cell-free mtDNA, ccf-mtDNA), triggering inflammatory signaling.
Increasing evidence indicates that BD is a systemic disorder affecting not only the brain but also peripheral systems. This leads to systemic comorbidities that may result from cumulative "wear and tear" from long-term exposure to stress. As a result, these comorbidities can influence and further deteriorate brain function, contributing to accelerated aging and neuroprogression. Our preliminary data have shown that BD patients had an imbalance in mitochondrial fission and fusion towards fission, downregulation of mitophagy-associated proteins, followed by cell death pathway activation. Still, the downstream effects on the mitochondrial bioenergetics and ccf-mtDNA are unknown.
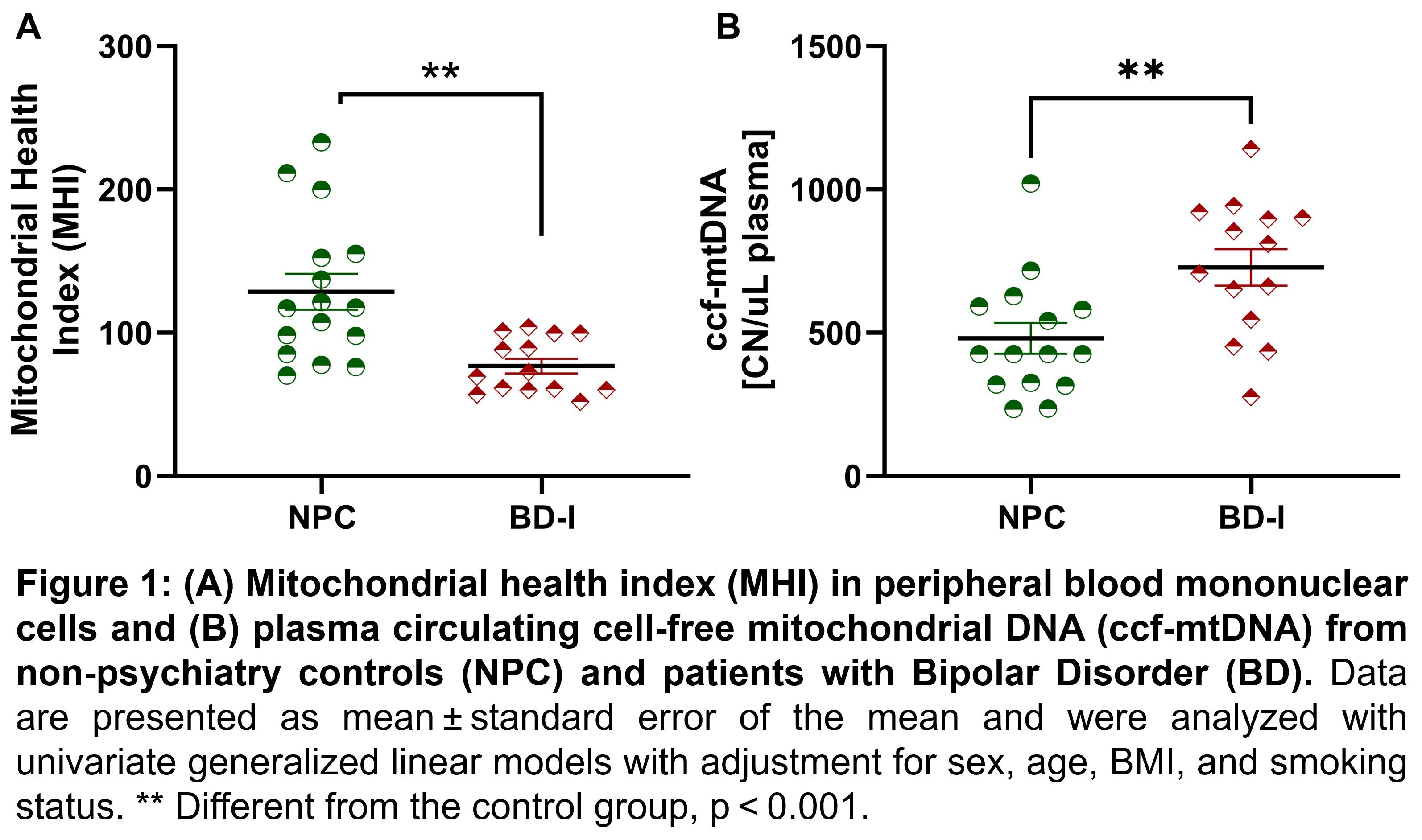
This led us to question: does the impairment in the mitochondrial quality control system mediate the decrease in mitochondrial bioenergetic and release of mitochondrial damage-associated molecular patterns (e.g., ccf-mtDNA) in BD patients? To answer this, we used the mitochondrial health index (MHI), a metric that integrates nuclear and mitochondrial DNA-encoded respiratory chain enzymatic activities and mtDNA copy number into an index reflecting mitochondrial respiratory chain capacity per mitochondrion basis. Our groundbreaking research sheds light on the relationship between bipolar disorder and mitochondrial health. Our findings indicate that even after adjusting for confounding variables, patients with bipolar disorder have lower MHI than non-psychiatry controls, which suggests a lower mitochondrial bioenergetic capacity. We also observed a positive correlation between MHI and increased mitochondrial fusion, followed by a lack of mitophagy activation, shifting the cell signaling to apoptosis activation. In response to cell death, mtDNA fragments are released into circulation, initiating pro-inflammatory responses. Our study also discovered that BD patients have a higher likelihood of experiencing perturbations leading to mitochondrial dysfunction, as evidenced by the correlation between MHI and ccf-mtDNA levels in BD patients but not in the control group (Figure 1). Additionally, our research underscores that MHI and ccf-mtDNA levels can predict depressive symptoms and functional impairment.
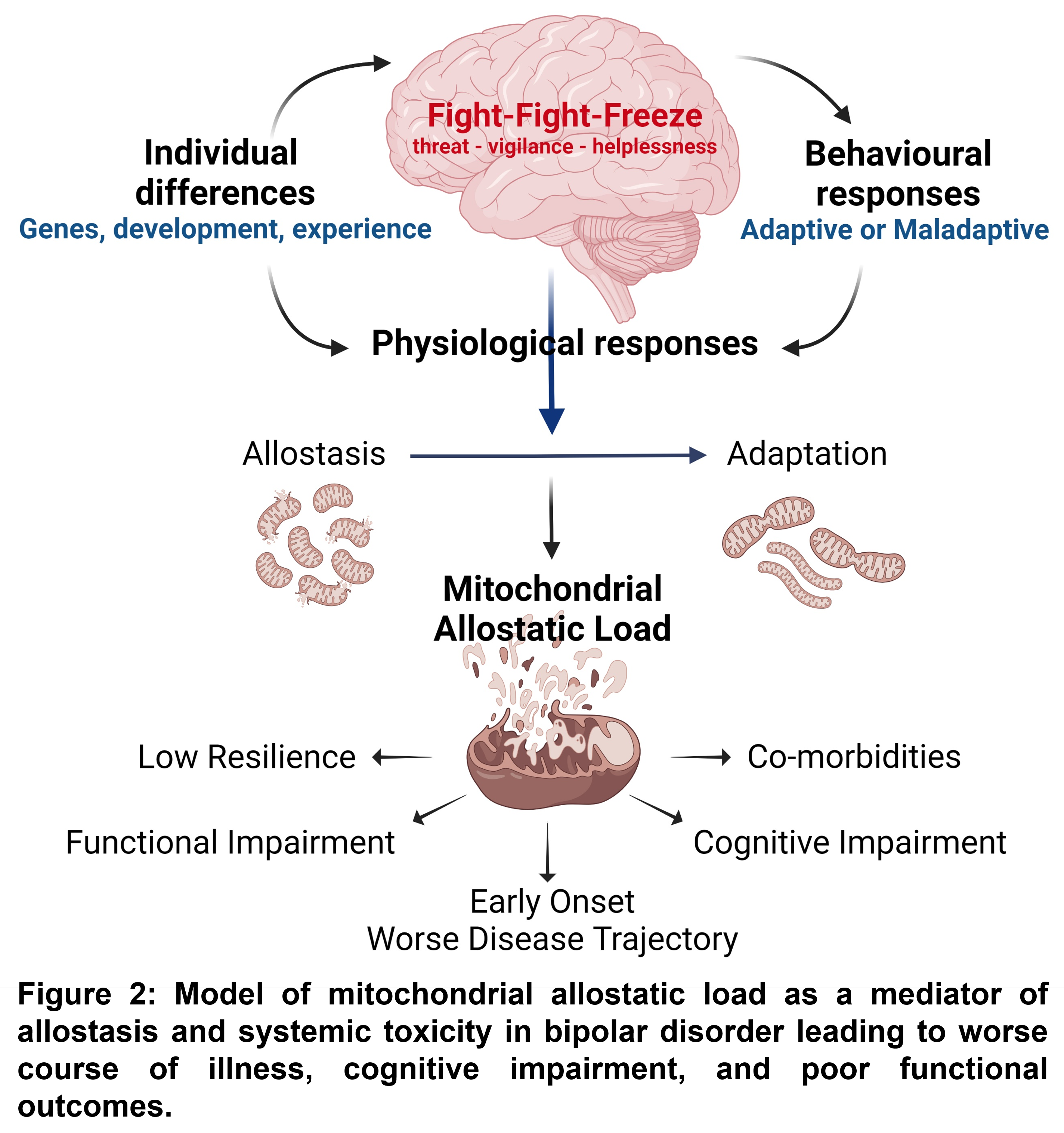
Patients with BD have complex changes in their mitochondrial biology, which can lead to an accumulation of stress (MAL) in these organelles. This can cause the disease to worsen and lead to poor outcomes through various mechanisms such as changes in gene expression and epigenomics, alterations in brain function and structure, abnormal reactions to stress, inflammation, and cellular aging. Thus, the development of BD might be linked to metabolic perturbations, suggesting that mitochondria may be a biological intersection contributing to reduced cellular resilience and increased vulnerability to stress and mood disorders since mitochondrial energy production determines the ability of an organism to adapt to stress (Figure 2). Understanding how mitochondrial dysfunction affects BD progression and outcomes could give us a predictive marker. It may also help us comprehend how mitochondrial function and regulation are related to BD and eventually create a guide for treatment with new and old therapeutic targets.
Follow the Topic
-
Molecular Psychiatry

This journal publishes work aimed at elucidating biological mechanisms underlying psychiatric disorders and their treatment, with emphasis on studies at the interface of pre-clinical and clinical research.
Your space to connect: The Psychedelics Hub
A new Communities’ space to connect, collaborate, and explore research on Psychotherapy, Clinical Psychology, and Neuroscience!
Continue reading announcement




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in