All-perovskite photoelectrochemical system for solar H2 at scale
Published in Chemistry
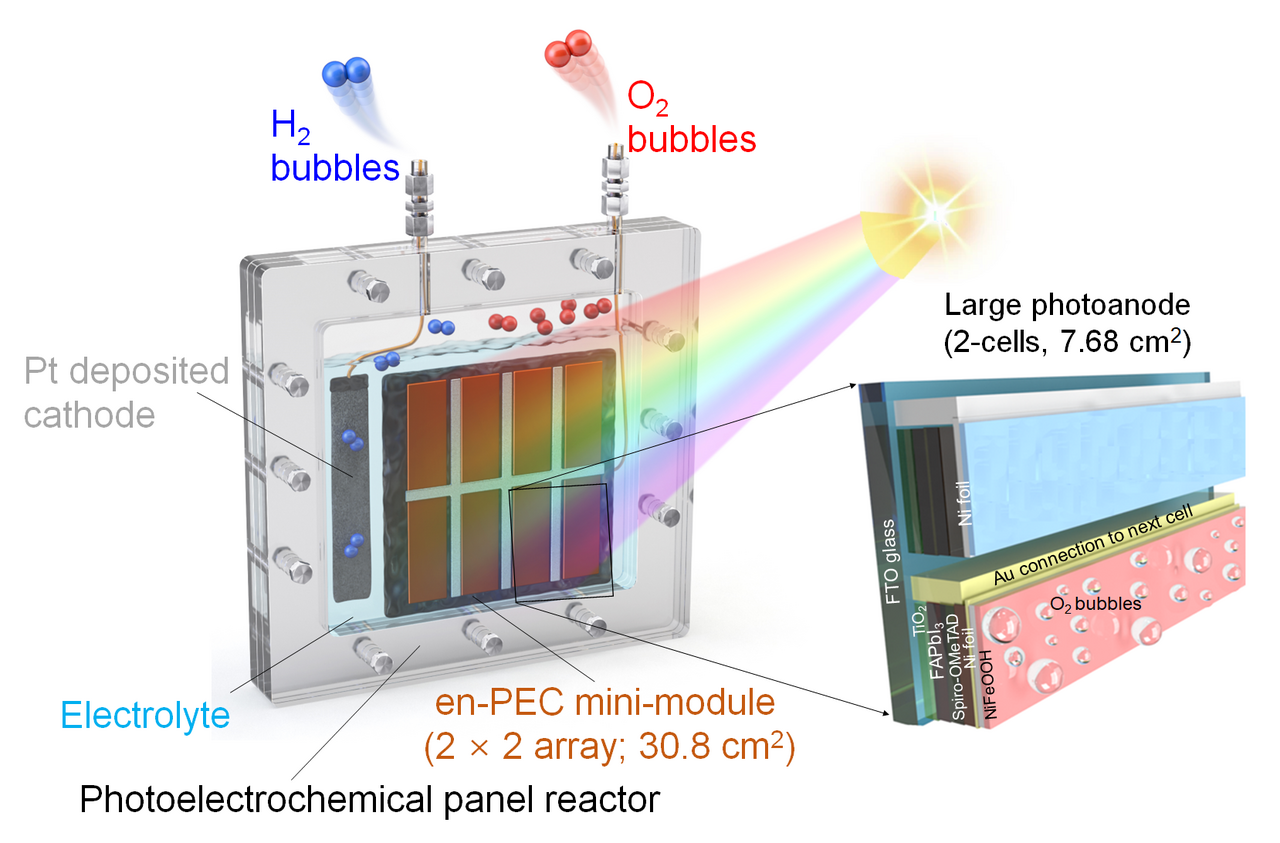
Scalable photoelectrochemical system demonstration for solar H2 at outdoor condition
Green hydrogen (H2) production from water and sunlight is of significant interest for fulfilling the world’s energy demand and carbon-rich pollution control on Earth. Amongst the various types of methods involved in solar H2 production [1-2], photoelectrochemical (PEC) water splitting is one of the carbon footprint-free technologies for artificial photosynthesis. A solar-to-hydrogen (STH) efficiency of at least > 10% is necessary for realizing such a practical PEC system in outdoor field conditions. Not only the high efficiency but stability and scalability of the PEC systems are also prime requirements.
Practical PEC systems have been investigated intensively due to the confined integration of large-area photoelectrodes for producing H2 by just utilizing sunlight and water. Yet, several scalability challenges are a concern such as material, its large-area fabrication, low-cost method, long-term durability, and efficiency. In the past few years, efforts have been reported for upscaling the PEC systems using representative materials including BiVO4, Fe2O3, WO3, LaTiO2N, and Cu2O large photoelectrodes [3-6]. However, most large-area photoelectrodes report a dramatically low performance compared to those small-area ones made from the same material. For example, BiVO4 shows high STH efficiency in the range of 6~8 % for small-sized (<0.3 cm2) [3-6], however, large-size BiVO4 photoelectrodes (1~50 cm2) showed an STH efficiency of only 0.1~3 %; which is 200~1000% decrement compared to that of small-size. So, realizing large-size PEC devices with high efficiency and stability could face challenges such as non-uniform thickness during upscaling thin films, reproducibility, efficiency, and long-term stability.
Practically viable PEC technology can be attained by selecting proper materials with high efficiency, scalability, and stability as well [1]. As the most promising next-generation photovoltaic (PV) materials, organic-inorganic hybrid perovskites (PSK) draw attention as potential photoelectrode material for PEC water splitting owing to their excellent optoelectronic, charge transport properties and scalability. However, their instability in humidity and poor photostability of conventional PSK materials were critical challenges for PEC applications, where the photoelectrode has to be immersed in water under solar irradiation [2,7].
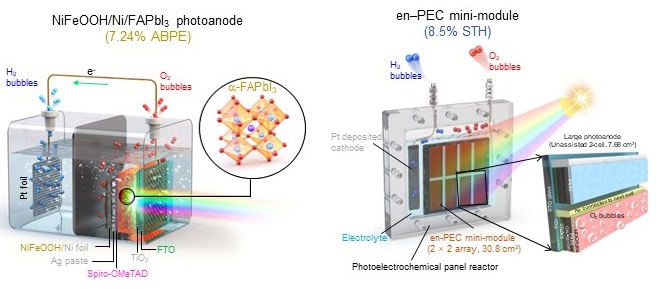
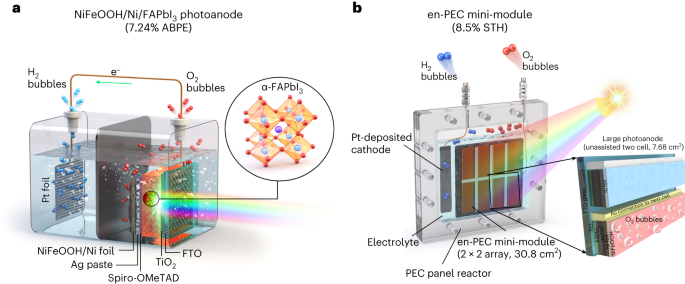
In our work, we apply efficient, crystalline, and intrinsically stable formamidinium lead triiodide (FAPbI3) as a light absorber to develop metal–encapsulated PSK photoelectrodes by passivating it with pinhole-free Ni foil loaded with low-cost NiFeOOH. We demonstrate a FAPbI3 perovskite-based photoanode of such potential, which is encapsulated by Ni foil deposited with a NiFeOOH electrocatalyst for oxygen evolution reaction. This metal–encapsulated FAPbI3 photoanode records a photocurrent density of 22.82 mA cm−2 at 1.23 VRHE and shows excellent stability for three days under simulated 1-sun illumination. We further construct an all-perovskite-based unassisted PECwater splitting system by connecting the photoanode with the same size FAPbI3 solar cell in parallel, which records a new benchmark of solar-to-hydrogen (STH) efficiency of 9.8%. We also demonstrate the scale-up of these Ni–Ni-encapsulated FAPbI3 photoanodes (mini-modules up to 123.2cm2) that record the STH efficiency of over 8.5%, which is the highest among all the reported large area photoanodes over 1 cm2 in unassisted solar water splitting.
For more details, please check out our paper “All-perovskite-based unassisted photoelectrochemical water splitting system for efficient, stable and scalable solar hydrogen production” in Nature Energy (2024).
Links to cite the article: https://dx.doi.org/10.1038/s41560-023-01438-x
https://www.nature.com/articles/s41560-023-01438-x
References:
[1] Kim, J. H. et al., Chem. Soc. Rev., 48, 1908 (2019)
[2] Yang, W. et al., Chem. Soc. Rev., 48, 4979 (2019)
[3] Huang M. et al., J. Mater. Chem. A, 8, 3845 (2020)
[4] Qayum A. et al., J. Mater. Chem. A, 8, 10989 (2020)
[5] Vilanova A. et al., J. Pow. Sour., 398, 224 (2018)
[6] Ahmet I.Y. et al., Sustain. Energy Fuels 3, 2366 (2019)
[7] Chen, J., Adv. Energy Mater., 10, 1902433, (2019)
Follow the Topic
-
Nature Portfolio Webcasts

-
Nature Energy

Publishing monthly, this journal is dedicated to exploring all aspects of this on-going discussion, from the generation and storage of energy, to its distribution and management, the needs and demands of the different actors, and the impacts that energy technologies and policies have on societies.
Related Collections
With Collections, you can get published faster and increase your visibility.
Microgrids and Distributed Energy Systems
Publishing Model: Hybrid
Deadline: Mar 31, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in
More information: Dharmesh Hansora et al, All-perovskite-based unassisted photoelectrochemical water splitting system for efficient, stable and scalable solar hydrogen production, Nature Energy (2024).
Link of Article
DOI: 10.1038/s41560-023-01438-x
https://dx.doi.org/10.1038/s41560-023-01438-x
https://www.nature.com/articles/s41560-023-01438-x