Scalable photoelectrochemical mini-module
Key Messages
-
The authors report a perovskite-based artificial leaf (i.e. a fully integrated, wireless photoelectrochemical device) that meets all three of the major criteria for practical solar water-splitting:
-
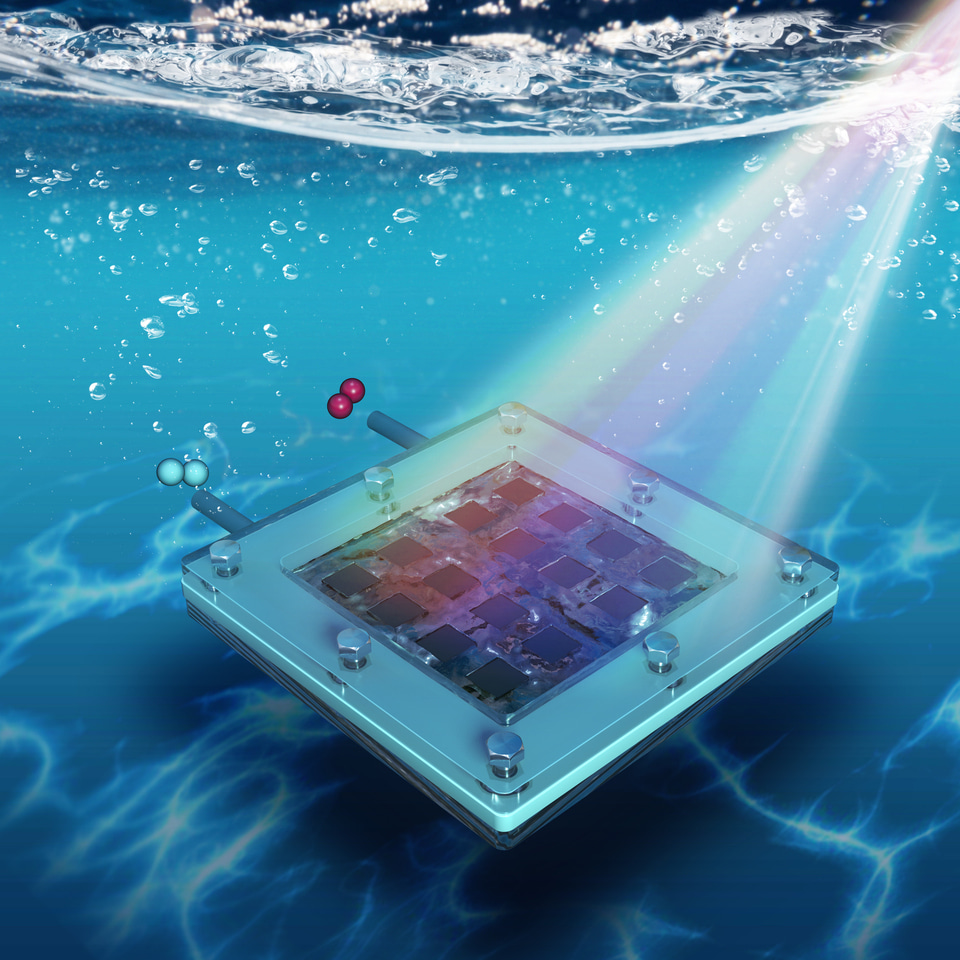
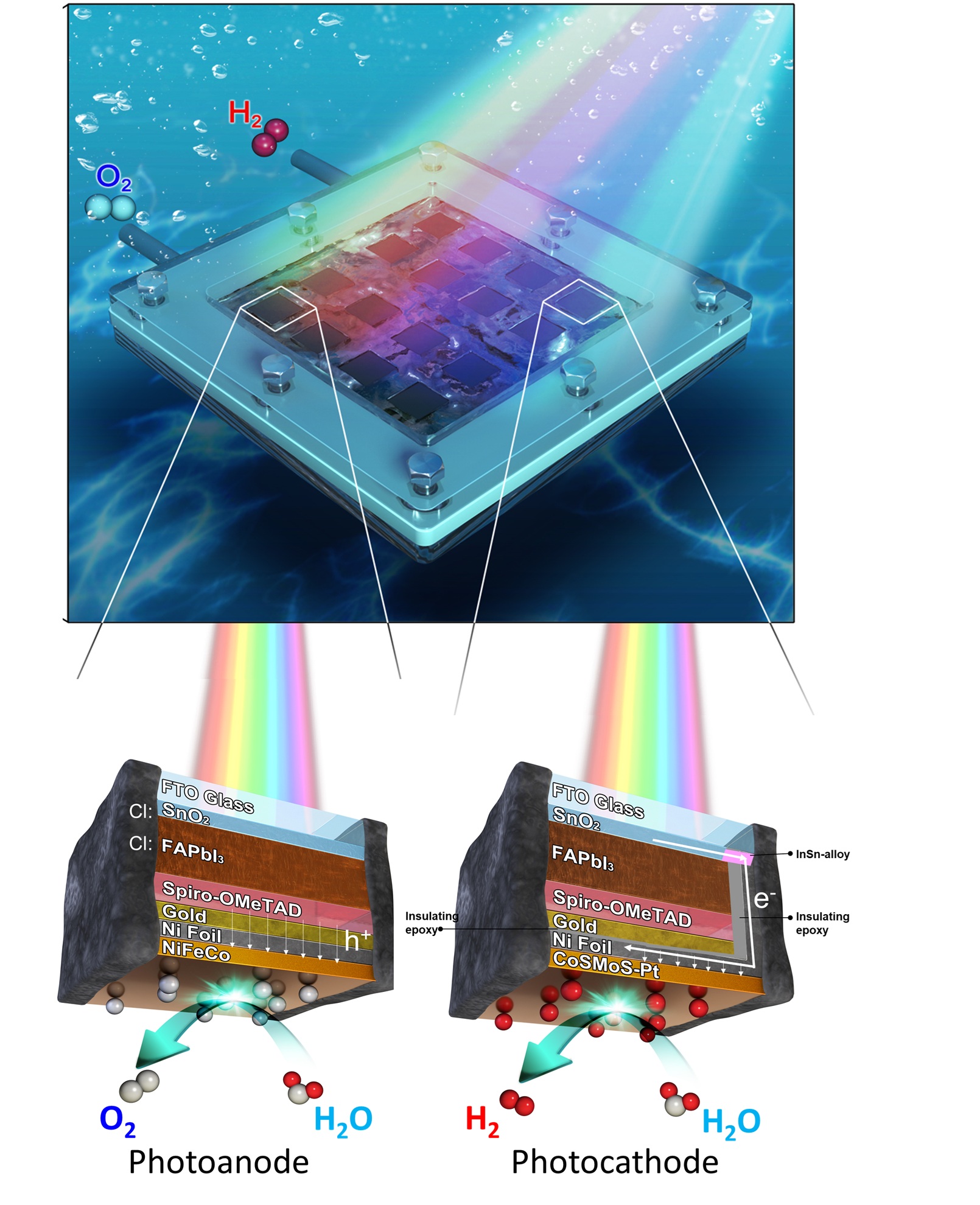
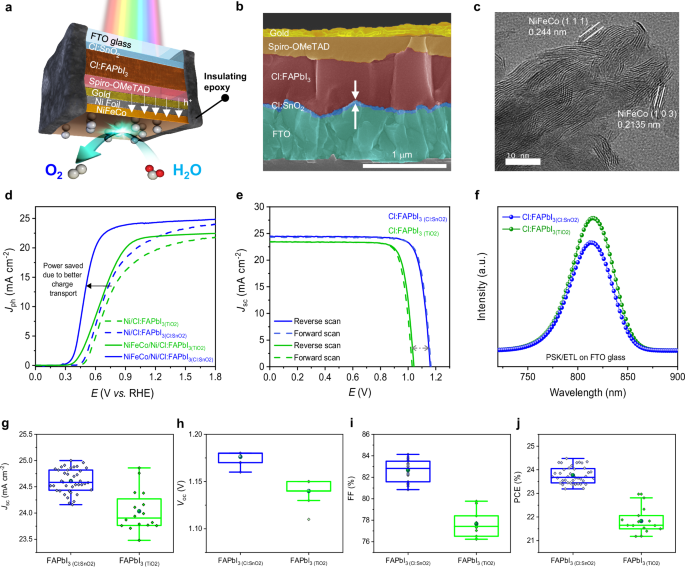
They build photoelectrodes using a defect-free, chlorine-doped formamidinium lead triiodide (Cl:FAPbI₃) as the light absorber and a chlorine-doped tin oxide (Cl:SnO₂) electron transport layer (ETL) that is more resistant to ultraviolet (UV) damage.
-
Encapsulation is handled with nickel foil and epoxy/other protective layers to shield the perovskite from electrolyte contact. The catalysis (for oxygen evolution, and hydrogen evolution) uses less expensive materials; for example, the oxygen evolution catalyst is NiFeCo oxyhydroxide, and the hydrogen evolution catalyst is a cobalt-molybdenum-sulfide mixture doped with a small amount of Pt (~0.1 wt%) for enhancement.
Key Results
-
At the 1 cm² device scale (photoanode), the device shows high photocurrent density and retains 99% of its initial performance after 140 hours of continuous operation in alkaline electrolyte. No lead leaching, and the perovskite absorber remains intact.
-
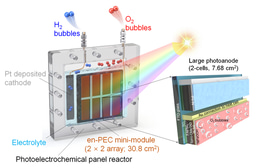
They assemble a mini-module artificial leaf, comprising a 4×4 array of 1 cm² subcells (total 16 cm²), arranged side-by-side with both photoanodes and photocathodes (See above schematic). This achieves a module-level unbiased STH efficiency of 11.2% under 1-sun illumination.
-
Scaling up from 1 cm² to the 16 cm² module incurs very small efficiency loss (≈ < 10%) showing good promise for further upscaling.
Innovations / Technical Advances
-
Use of Cl-doping both in the PSK absorber (FAPbI₃) and in the ETL (SnO₂): this helps improve stability & reduce defects.
-
Replacing UV-sensitive TiO₂ ETLs (common in many perovskite solar devices) with Cl:SnO₂ to reduce UV-induced degradation.
-
Effective encapsulation strategy and reactor design (e.g. O-ring type reactor that exposes only the catalysis layer to electrolyte; prevents perovskite or epoxy being in direct contact) to avoid chemical/photocorrosive degradation.
-
Using non-noble-metal catalysts (or very low loadings of noble metals) to keep cost and material constraints more favorable.
Implications & Challenges
-
This work marks a step closer to commercial viability for artificial leaves: achieving >10 % STH at module size with long durability is rare.
-
Because of the modular design, the authors argue that further scaling (towards meter-scale, comparable to current solar panels) could preserve similar efficiencies.
-
Remaining challenges include:
• Ensuring efficient proton transport between the anode and cathode, especially in larger modules,
• Managing gas separation (H₂ and O₂) in a side-by-side architecture (currently they occupy the same electrolyte side), which may require membranes or external separators.
For more details, please check out our paper “Scalable and durable module-sized artificial leaf with a solar-to-hydrogen efficiency over 10%” in Nature Communications (2025).
Links to cite the article: https://dx.doi.org/10.1038/s41467-025-59597-2
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in