
Perovskite-type oxynitrides became promising candidates for red and orange pigments in 2000 and with this also for visible-light driven processes. Despite, the large research efforts in our group and other research labs all over the world a remaining challenge is to control the O:N ratio of the product, because the formation mechanism is still a matter of debate. In particular, the conversion of nanocrystalline oxide precursors during ammonolysis to defined oxynitrides is yet unknown. The knowledge of the fundamental reaction steps is crucial to be able to synthesize oxynitride materials with suitable composition and properties.
For a reproducible synthesis of homogeneous products precisely controlled nitridation via known intermediates is a prerequisite.
During our studies we recognized that the microstructure is a crucial selection criterion defining which reaction pathway during ammonolysis is used to convert the oxide precursors to an oxynitride. This became obvious during our in situ thermogravimetric analysis (TGA) in flowing ammonia. This very powerful method allows to monitor temperature- and time-resolved the progression of a chemical reaction with a mass change. We supported these in situ measurements by termination experiments and in-depth analysis of the received intermediate products by chemical analysis and X-ray photoelectron spectroscopy (XPS).
We tested the reaction behavior of two precursors with different microstructures. Both showed a clearly different reaction progression leading to two distinct products, namely bright-red before unknown LaTaO2N and purple LaTaON2. The first one is of special interest since it contains tantalum in the unusual oxidation state of +4. LaTaO2N combines the presence of unpaired 5d1 electrons with an intense bright-red color, while such materials e.g. VO2 typically show a black or bluish color.
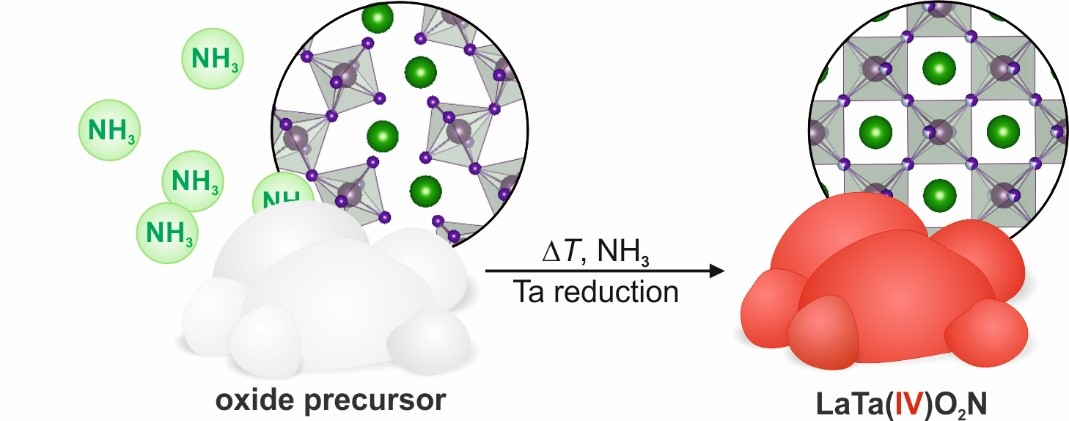
Figure 1: Reaction scheme of the conversion of an oxide precursor to LaTa(IV)O2N
This study added the precursor microstructure as another important control parameter besides the classical ones such as reaction temperature and time, gas flow rate, and ammonia concentration to the toolbox of chemists and material scientists to synthesize further new oxynitride phases. If we have awakened your interest in the synthesis of colorful oxynitrides we recommend to consult our publication in Communications Chemistry.
Tailoring of an unusual oxidation state in a lanthanum tantalum(IV) oxynitride via precursor microstructure design
DOI: https://doi.org/10.1038/s42004-019-0237-x
*This post was co-authored by Cora Bubeck, Dr. Marc Widenmeyer, and Prof. Anke Weidenkaff.
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in