Predicting Human Brain Temperature
Published in Electrical & Electronic Engineering
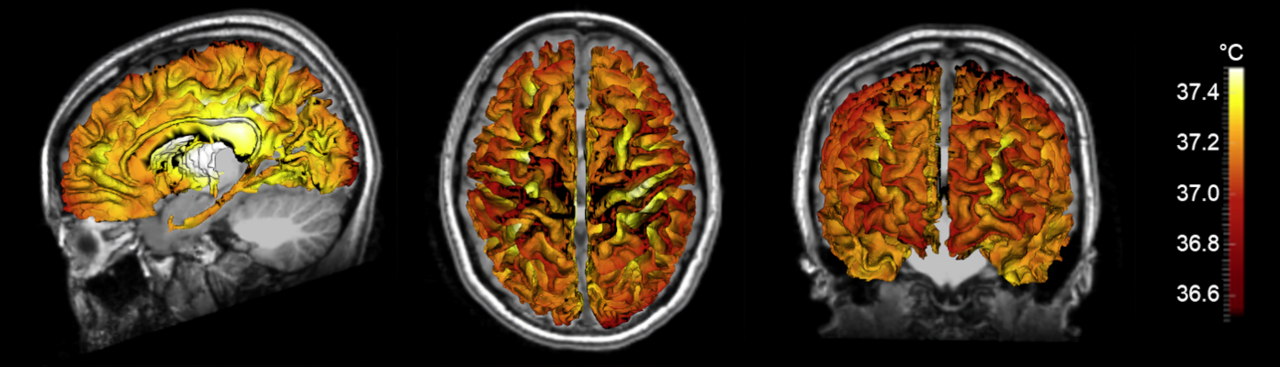
While brain temperature measurements were first reported as early as the mid-nineteenth century, our understanding of human brain temperature is still largely limited by a lack of non-invasive thermometry methods. Recent advances in magnetic resonance imaging (MRI) technology have facilitated whole brain temperature measurements via chemical shift thermometry, but several challenges limit routine clinical application. Given the importance of brain temperature regulation for recovery after stroke, heart attack, and head injury, there is an immediate need to characterize thermal dynamics in the individual brain.
For the past several years the Fleischer Lab, led by Dr. Candace Fleischer at the Emory University School of Medicine, has been developing non-invasive methods for quantitative and absolute brain thermometry. Together with clinical collaborators Dr. Jason Allen and Dr. Fadi Nahab, and biostatistician Dr. Benjamin Risk, our team previously demonstrated non-invasive MR brain thermometry in a primate stroke model and in patients with cerebrovascular disease (AJNR, 2017;38(11):2044; AJNR, 2017, 38(4):712). We observed small increases in temperature are associated with measurably worse outcomes, confirming the role of brain temperature as a potential prognostic marker after injury. While these findings were promising, a limitation of current brain thermometry is the lack of external validation. Implanted temperature probes used in surgery are impractical for research use, and there are currently no routine methods to measure and predict temperature in patients.
To address the challenges in experimental thermometry, predictive biophysical models have been developed to characterize human brain temperature. Most models rely on fundamental principles of bioheat transfer, e.g., Pennes’ bioheat transfer equation (J Appl Physiol, 1948;1(2):93), and progress has been made in generating realistic human anatomy (Phys Med Biol, 1996;41(5):865; Sci Rep, 2018;8(1):7877). Absent from current models, however, is 1) full conservation of energy, mass, and momentum when calculating temperature, and 2) personalized predictions of brain temperature accounting for differences in brain anatomy between individuals. As personalization, or tailoring treatment and care for each patient, is an important goal of contemporary medicine, individual predictions are crucial.
With the goal of developing an improved biophysical model of human brain temperature, our team collaborated with Dr. Andrei Fedorov, an expert in the field of heat transfer and first principles biophysical modeling. Our current manuscript addresses many of the deficiencies in previous models, including adherence to first principles of heat transfer physics as well as the use of individual brain data for personalized predictions. Biophysical modeling using a 3D formulation of thermal physics in the brain by Dongsuk Sung, a graduate student in Dr. Fleischer’s laboratory, and Dr. Peter Kottke, a research engineer in Dr. Fedorov’s laboratory, combined with measured brain anatomy from each subject (brain tissue and vessel structure), enabled detailed predictions of individual brain temperature maps.
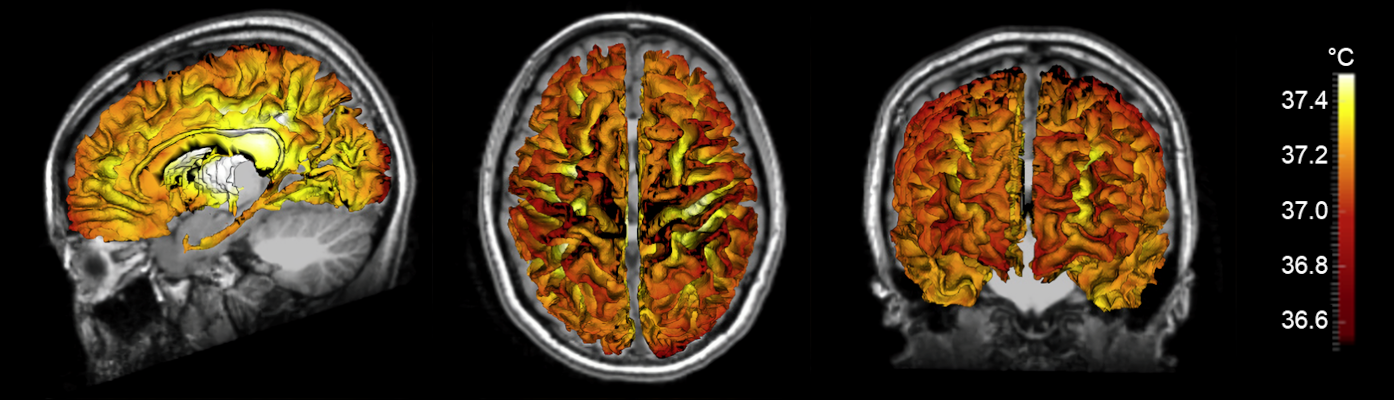
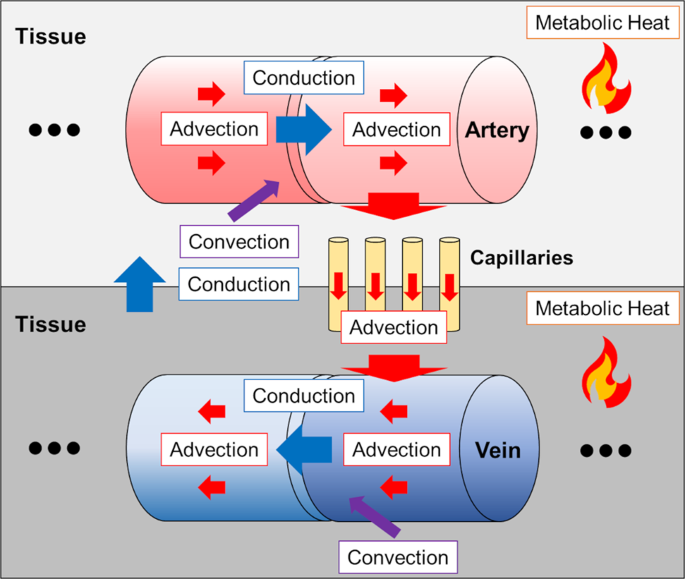
Our model accounts for heat transfer within the brain via conduction, convection, and advection, and was calculated in three physical domains: arteries, tissue, and veins. Individual vessel and tissue structure was obtained from MRI data collected from each subject, and vasculature was augmented using a randomly exploring rapid tree algorithm. Vessel structure maps were used to generate flow rates throughout the blood vessel network, as cerebral blood flow is the primary mechanism for heat dissipation in the brain. Heat generation is the result of cerebral metabolism, and tissue structure maps were used in a similar manner to calculate local metabolic rates. Finally, steady-state equations for each domain were formulated to ensure local and global energy conservation and solved to calculate 3D temperature distributions. The primary finding of this study was the observation that temperature magnitude and spatial patterns differ between individuals, further supporting the need for a personalized approach to brain temperature management. Our modeling efforts were accompanied by experimental MR brain thermometry in healthy humans, and temperature measurements were similar to model predictions.
Building on these foundational results, future work will simulate the thermal response of the brain in patients after injury such as ischemic stroke. We anticipate brain temperature predictions and measurements will facilitate improved prognostication and patient stratification for emerging treatments, particularly after cerebrovascular injury. Our full manuscript published in Communications Physics is available here.
Follow the Topic
-
Communications Physics

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the physical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Physics-Informed Machine Learning
Publishing Model: Hybrid
Deadline: May 31, 2026
Advances in Quantum Networking: QKD and beyond
Publishing Model: Open Access
Deadline: Apr 30, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in