Pumping liquid tin at 1400°C
Published in Chemistry

The P-block elements of the periodic table are fascinating in the sense that, as their atomic number increases, the properties of the elements in the same column change far more significantly compared to the rest of the periodic table. Column 14, which contains both tin and carbon for example, has nonmetals at the top, metalloids in the middle, and post-transition metals at the bottom. The conventional wisdom, which is based on the rest of the periodic table, suggests that elements in the same column would have a similar electronic configuration and therefore would behave in a chemically similar way. For example, one might think that by being in the same column as carbon, the rest of the elements in column 14 might possess significant solubility or reactivity towards carbon. However, as a general trend, the reactivity of the elements decreases as the atomic number increases. Thus, among them, only Si and Ge react with C to covalently to form carbides, although even Si does not show mutual solubility with C. The rest of the elements in this group Sn and Pb not only do not form stable carbides, they also show negligible solubility or even wetting (ϴ>120°) with carbon.
Specifically of interest in our work, tin does not wet, dissolve, or react with graphite appreciably at temperatures below its boiling point. Fundamentally, this is because while forming a tin-carbon solution would be a chemical reaction with intrinsic increase in entropy, this reaction can only happen if the entropic contribution to Gibbs energy exceeds the enthalpic portion. In this case, the increase in entropy for forming a solution does not overcome the enthalpic barrier. This is due to the very strong covalent C-C bond in carbon, manifested by the deep, narrow potential well which would likely be displaced greatly by large Sn atoms (more than 2X the diameter). The first step in making this solution would be substituting the existing C-C and Sn-Sn by Sn-C bonds. However, the very strong C-C bonds cause the total dissolution reaction to be very endothermic. This also results in the very high dissociation temperature for different allotropies of carbon, e.g. diamond and graphite. Graphite for example does not melt but sublimes at around 3642°C, while on the other hand tin melts at 232°C, which is an extreme contrast. Simply put, C and Sn are so different that they cannot chemically interact with each other at all, even though they both belong to the same group of the periodic table. This is also manifested with their simple phase diagram which shows no compound or solubility regions at any temperature. The refractory nature of graphite, and its resistance to bonding with tin, combined with tin’s low melting point of only 232°C and high boiling point of 2,602°C make them a perfect couple as refractory container and heat transfer fluid.
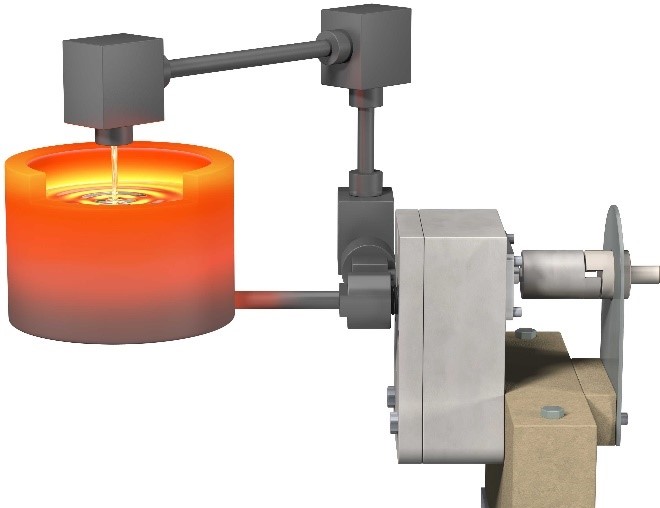
Even though there are other elements (e.g. Ga, In, Pb) that are chemically compatible with graphite and good thermophysical properties for a heat transfer fluid (high thermal conductivity, low viscosity), Sn is the best candidate because it is relatively low cost and non-toxic. The affordable cost of Sn is the result of several factors: its abundance, concentration in ore, low chemical affinity with oxygen, and long history of use by humans. In fact, tin was discovered more than 4000 years ago and has had various uses since. For example, bronze, which is a copper-tin alloy, had such an important impact on human history that it has a period of human history named after it (i.e., “the bronze age”). Today, tin is often used for solder, corrosion-resistant coatings, ductile alloys, and glass making. It is in large part due to this wide range of uses, in addition to high concentration regions of Cassiterite (SnO2 ore) that result in tin’s affordable cost of ~$20/kg. Furthermore, tin does not react with many refractory materials, including alumina, aluminum nitride, boron nitride, silicon carbide, and even tungsten. Thus, due to scientific and economic considerations, in our recent paper (http://dx.doi.org/10.1038/nature24054) we demonstrated the use of graphite and other refractory ceramics and metals to contain, seal, and pump tin at up to 1400°C. An illustration and image of this experiment is shown below, where an all graphite piping and sealing network was used, in addition to an AlN-BN pump, one of the many ceramics that tin is compatible with.

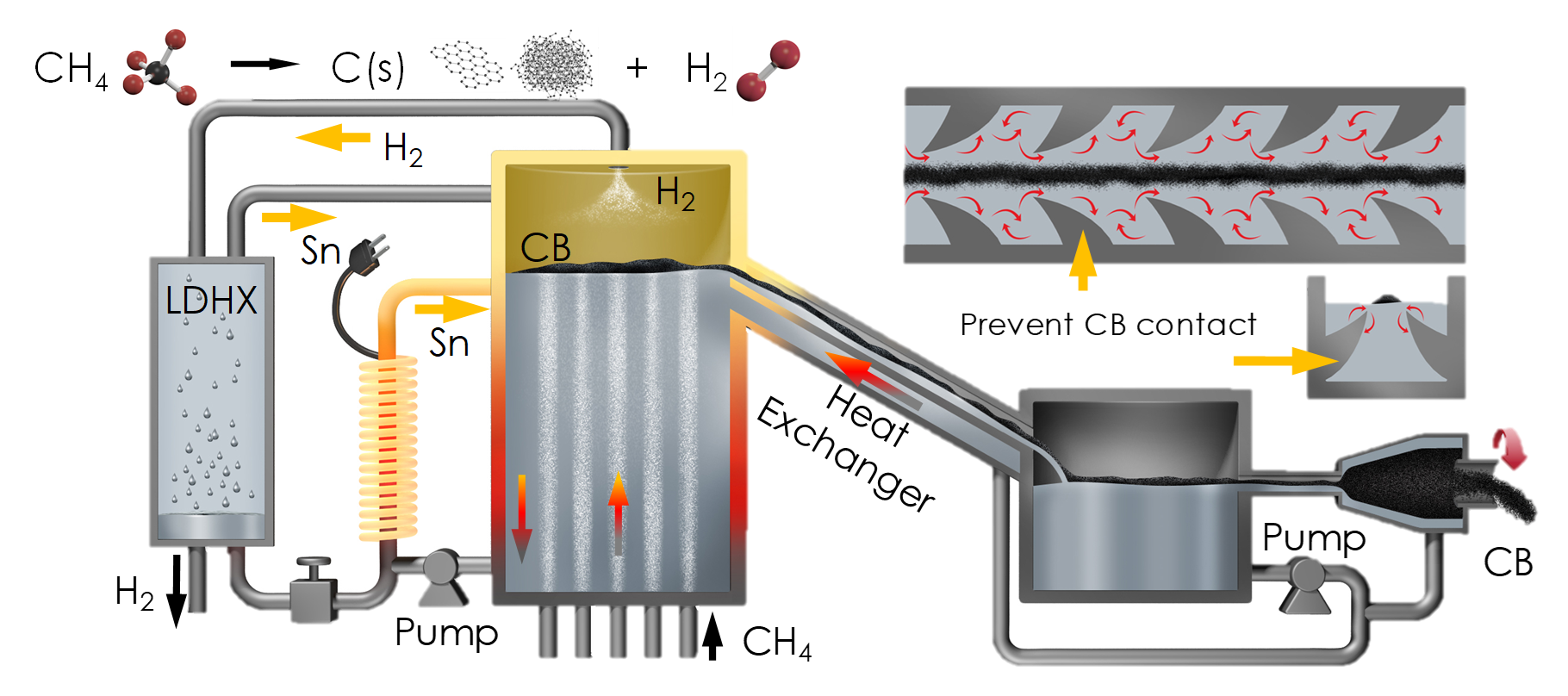
There are many applications of interest for the use of tin as a heat transfer fluid, ranging from ultra-high temperature and high efficiency concentrated solar power, to high temperature waste heat recovery (e.g. in aluminum production). One timely application is the production of hydrogen via methane cracking, or pyrolysis. This application is particularly important as society begins to focus on reducing emissions beyond the electricity sector. A critical difference between methane pyrolysis and the dominant method of hydrogen production, methane reforming is that carbon leaves as a solid instead of bonded to oxygen as CO2. A key technical issue in this approach is that the solid carbon tends to deposit on all solid surfaces and quickly clogs the reactor. A novel approach to resolving this issue is to keep the carbon from reaching solid surfaces until it is cooled. This can theoretically be achieved by transporting it on the surface of pumped molten tin, where its thermal energy is recuperated to preheat incoming methane, as indicated in the illustration below. Some aspects of this approach have been demonstrated by researchers at Karlsruhe Institute of Technology. Similarly, at Georgia Tech and MIT, we have already demonstrated many of the technologies involved in this concept, and at even higher temperatures (1400°C) than required for methane cracking (~1000°C).
Follow the Topic
-
Nature

A weekly international journal publishing the finest peer-reviewed research in all fields of science and technology on the basis of its originality, importance, interdisciplinary interest, timeliness, accessibility, elegance and surprising conclusions.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in