Radiotherapy may enhance the efficacy of immune checkpoint inhibitors in non-small cell lung cancers harboring features of immunotherapy resistance: evidence of the abscopal effect.
Published in Cancer
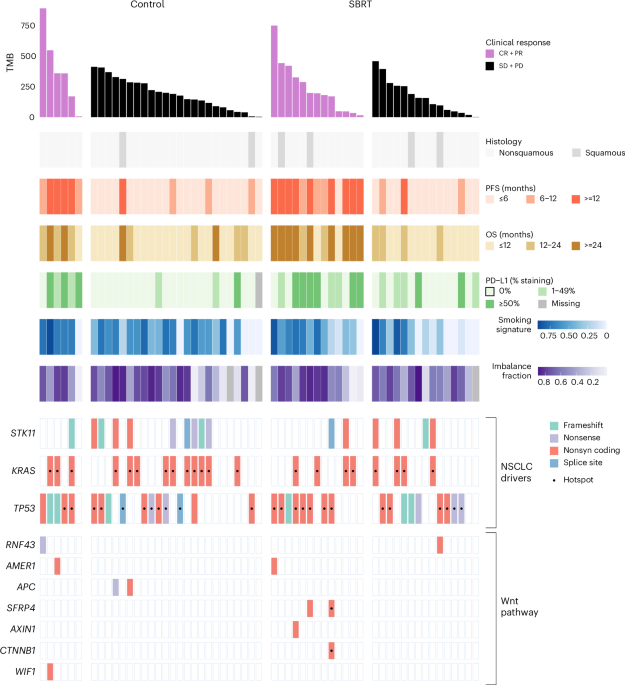
Lung cancer is the most deadly cancer worldwide. In recent years, the introduction of immune checkpoint inhibitors, especially anti-PD1/L1 treatment, has significantly improved the 5 year overall survival in advanced NSCLC. Unfortunately, subtypes that are referred to as immunologically cold tumors are often irresponsive to this treatment. Immunologically cold tumors can be characterized by a lack of immune cell infiltration, a low tumor mutational burden (TMB), null PD-L1 expression and mutations in the Wnt-pathway.
Radiotherapy has the ability to modulate the immune response against tumors through increased antigen release, improved antigen presentation and subsequent priming of new T-cells in the tumor draining lymph nodes. This immune activation can therefore induce systemic effects as shown by previous studies of tumor regression in non-irradiated lesions, referred to as abscopal effect. However, evidence of the abscopal effect is scant, as radiotherapy can also generate immune suppression, for example through the induction of PD-L1 expression on tumor cells. As such, the addition of immune checkpoint inhibitors may be able to boost this anti-tumor immune response, leading to clinically significant improvement in tumor response and long-term patient outcomes.
To test this hypothesis, we conducted the randomized phase II PEMBRO-RT trial (NCT02492568). Here, we compared outcomes of advanced NSCLC patients treated with either the anti-PD1 checkpoint inhibitor pembrolizumab alone (control arm) vs pembrolizumab dosed within one week after radiotherapy to a single tumor lesion (radio-immunotherapy arm). This was the first randomized trial showing significantly improved progression-free survival and overall survival in the radio-immunotherapy arm. Interestingly, this benefit seemed driven by patients harboring an immunologically cold tumor defined by null PD-L1 expression. Matched baseline and on-therapy tumor samples of non-irradiated tumor lesions together with longitudinal blood samples allowed us to capture the abscopal effect.
In our translational analyses of the PEMBRO-RT trial, we showed an induction of a systemic immune response with radio-immunotherapy especially in tumors harboring molecular features of immunotherapy resistance. In the radio-immunotherapy arm, response to treatment was seen irrespective of TMB or level of PD-L1 expression. These tumor characteristics are well-known predictors of response to pembrolizumab as was confirmed in the control arm of the study. In the radio-immunotherapy arm, upregulation of inflammatory pathways after initiation of pembrolizumab was significantly more pronounced compared to the control arm, especially in tumors with features of primary immunotherapy resistance. This was confirmed by induced influx of CD8+ T cells, M1 macrophages, CD4 memory and NK cells in this subgroup based on deconvolution of serial transcriptomic data. These findings support that the radio-immunotherapy may reshape the tumor microenvironment (TME) of abscopal tumor sites towards a more inflamed phenotype.
Interestingly, upregulation of a B cell gene signature seemed restricted to patients treated in the radio-immunotherapy arm. This may be a signal of induction of tertiary lymphoid structures (TLS), which is important element in the anti-tumor immune response. After further exploration, we found that a TLS gene expression signature was indeed significantly upregulated on-therapy, but specifically in PD-L1-null and Wnt-mutated tumors. These findings support the induction of systemic immune responses involving both T and B cell immunity by radio-immunotherapy and are again suggestive of a potential “cold-to-hot” conversion of the TME.
In our study, these findings were associated with improved patient outcomes. Long-term survivors in the radio-immunotherapy arm had a significant higher upregulation of inflammatory genes on-therapy compared to short-term responders, even if these long-term survivors harbored tumors at baseline with unfavorable prognostic characteristics, like a high proliferation and DNA replication phenotype. Also, response of the biopsied lesion according to RECIST measurements showed a more pronounced shrinkage in the radio-immunotherapy arm compared to the control arm and this was most pronounced in the subgroup of patients harboring tumors with molecular features of immunotherapy resistance. This was associated with benefit in overall survival.
Notably, the upregulated immune response in the radio-immunotherapy arm was also confirmed through reshaping of the TCR repertoire, where an increase of pre-existing and new T cell clones within the tumor as well as in the peripheral blood was more pronounced compared to the control arm, which was again irrespective of PD-L1 expression or TMB. In performing ex vivo autologous T cell testing, we were able to identify mutation associated neoantigen (MANA) reactive T cells in long-term several responders in the radio-immunotherapy arm.
Taken together, these analyses revealed an induction of systemic immune responses with radio-immunotherapy including in tumors harboring molecular features of immunotherapy resistance, likely driven by MANA cross-presentation in abscopal tumor sites. This study highlights the importance of a better understanding of the abscopal effect, as patient selection may drive the success of this treatment paradigm and the lack of it may explain the current mixed results of (pan-cancer) clinical trials in this field. Our findings support the immunomodulatory effects of radiotherapy, along with a pathway for radio-immunotherapy as a potential strategy to overcome primary resistance to immunotherapy in patients with immunologically cold tumors.
Follow the Topic
-
Nature Cancer

This journal aims to provide a unique forum through which the cancer community will learn about the latest, most significant cancer-related advances across the life, physical, applied and social sciences.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in