Real-world evidence of radium-223 utilization and associated outcomes can help guide treatment decisions in patients with metastatic prostate cancer
Published in Cancer and General & Internal Medicine
Why is it important to understand radium-223 utilization patterns and outcomes in advanced prostate cancer?
Radium-223 is a systemic radionuclide therapy approved for the treatment of metastatic castration-resistant prostate cancer (mCRPC) that has spread to the bones. Previous studies have shown that radium-223 is associated with prolonged survival and improved quality of life versus placebo. However, since it was approved in the United States in 2013, the treatment options available for men with mCRPC have expanded. Patients may now receive more life-prolonging therapies prior to their cancer becoming metastatic and castration resistant, such as hormone therapy, with or without chemotherapy. As a result, analyzing the utilization patterns and outcomes of radium-223 therapy in the real world may help clinicians decide when best to use radium-223 and whether it should be combined with other treatments.
How was the study conducted?
In this study, we used a large real-world dataset from US health insurance claims to examine the treatment patterns and overall survival (OS) of men with mCRPC treated with radium-223. This was a retrospective analysis of a cohort of men with prostate cancer who were treated with radium-223 (and therefore assumed to have mCRPC) between January 2017 and June 2022. All individuals required a baseline period of ≥12 months prior to first radium-223 administration and a follow-up period of ≥6 months (or until death if they died within 6 months of this date).
We assessed the rate of completion of ≥5 radium-223 cycles, the use of combination and layered treatment, and the sequence of prostate cancer therapies prior to and subsequent to radium-223. Completion of radium-223 therapy was defined as completing ≥5 cycles with gaps of <8 weeks between administrations. Combination therapy was considered the administration of other life-prolonging mCRPC medications within 30 days of starting radium-223, and layered therapy was considered as starting radium-223 after using another therapy for ≥30 days. Real-world OS was calculated as the time from radium-223 initiation to death.
What were the real-world utilization patterns of radium-223?
Of the 1376 men with mCRPC included in the analysis, we found that:
- Overall, 73%, 32%, 83%, and 74% of men had received prior androgen receptor pathway inhibitors (ARPIs), chemotherapy, opioids, and bone health agents, respectively
- Radium-223 was most commonly given as a second (35%) or third (25%) line of therapy
- Only 17% of men received radium-223 as first-line therapy
- Following radium-223 initiation, 45%, 19%, and 9% of men received one, two, or three subsequent lines of therapy, respectively
- Chemotherapy-ARPI and ARPI-chemotherapy were the most common line of therapy sequences
- Radium-223 was given as monotherapy, layered, or combination therapy in 64%, 28%, and 8% of men, respectively (Figure 1A)
- Androgen receptor inhibitors and abiraterone were the most commonly used agents in combination/layered radium-223 therapy (Figure 1B)
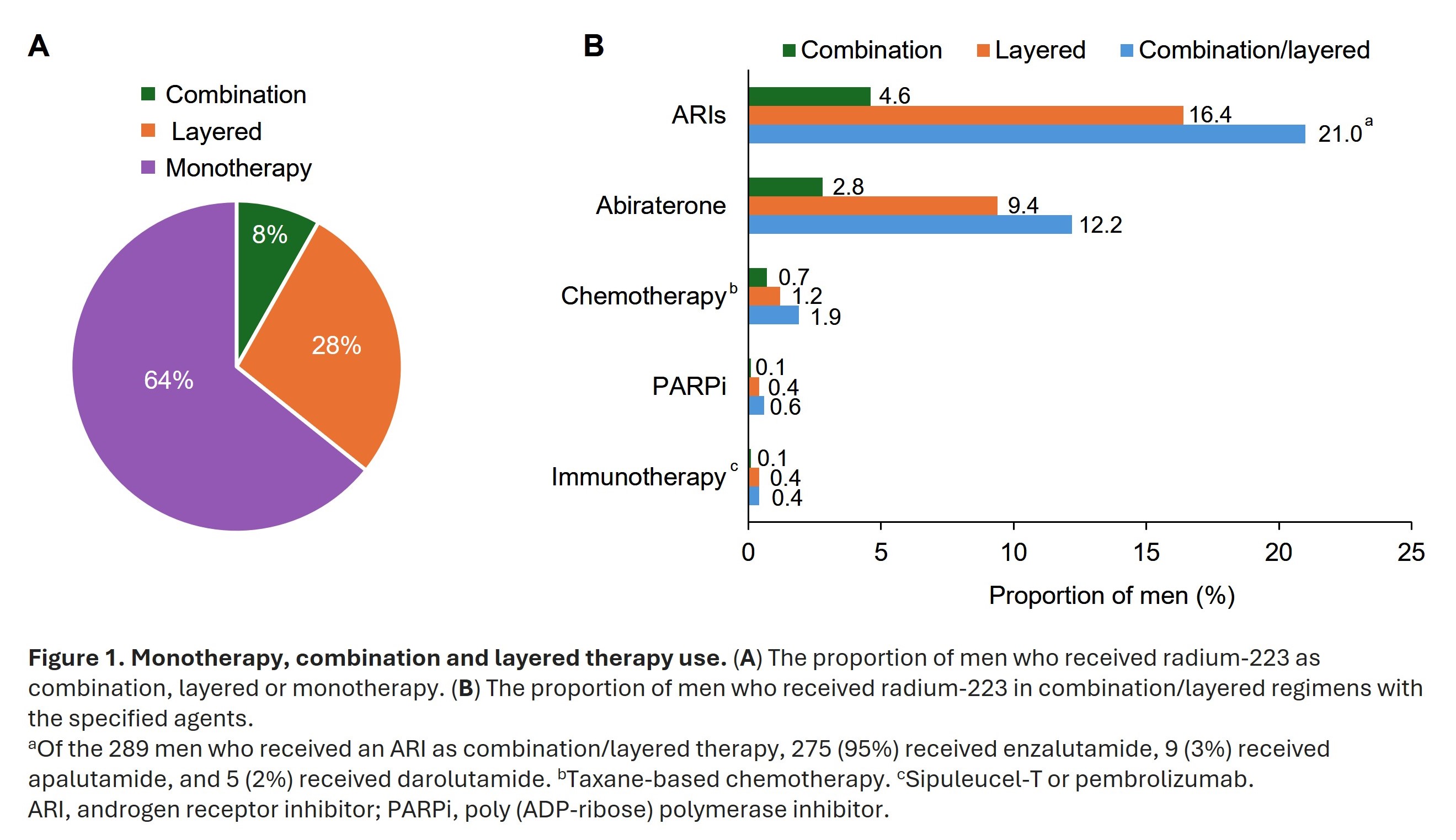
- Overall, 46% of men completed ≥5 radium-223 cycles. Men who were more likely to complete ≥5 radium-223 cycles included those who had metastases only in bone, received radium-223 as a combination or layered therapy, received radium-223 as first or second line of therapy, or had not previously received abiraterone, chemotherapy or opioids
What were the real-world survival benefits in men who received radium-223?
- Median real-world OS was longer in men who completed ≥5 radium-223 cycles versus 1–4 cycles (30.3 vs 15.3 months; Figure 2A) and in those who had combination/layered therapy versus monotherapy (26.6 vs 20.5 months; Figure 2B)
- These trends were similar whether radium-223 was used as first, second, third or fourth line of therapy
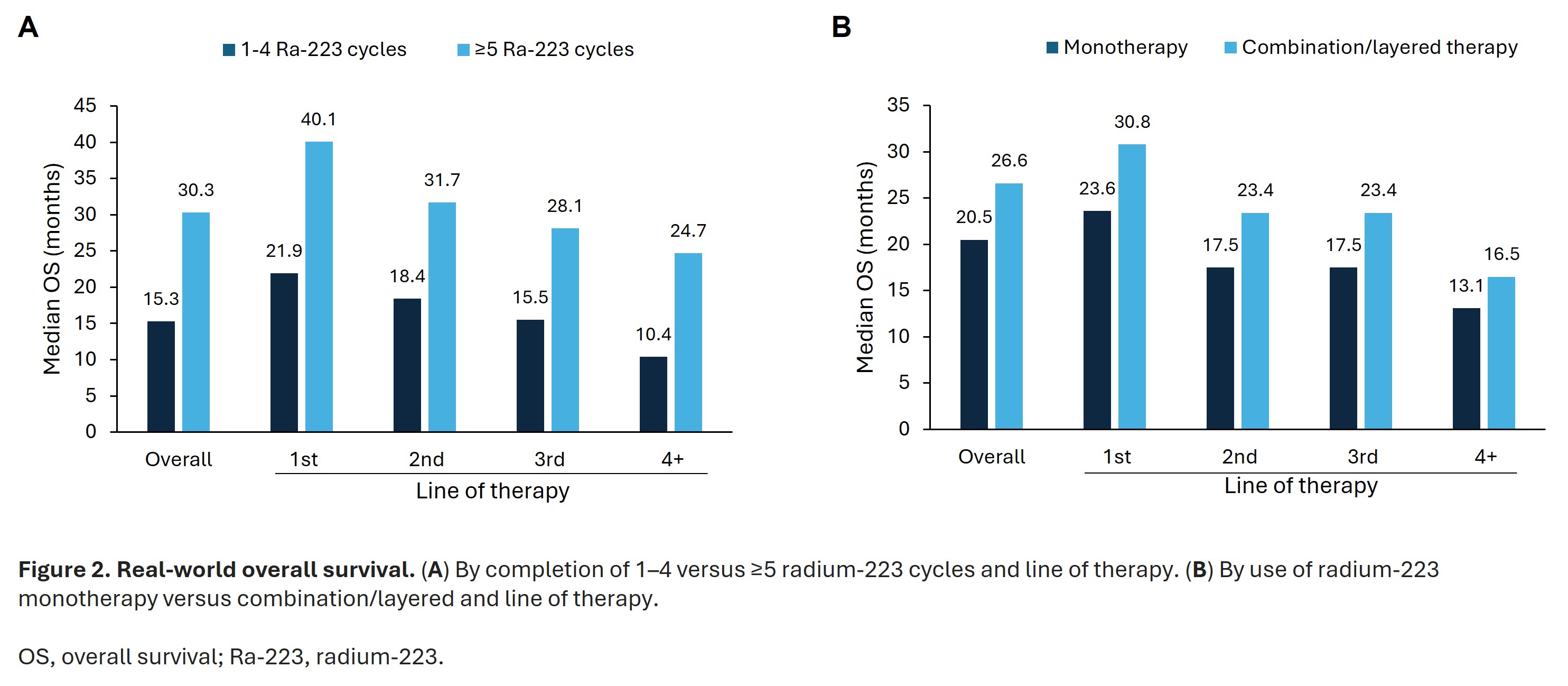
- For those completing ≥5 cycles of radium-223, the risk of death was 55% lower than those who completed 1–4 cycles
- Risk of death was 22% lower when radium-223 was used as a combination/layered therapy versus monotherapy
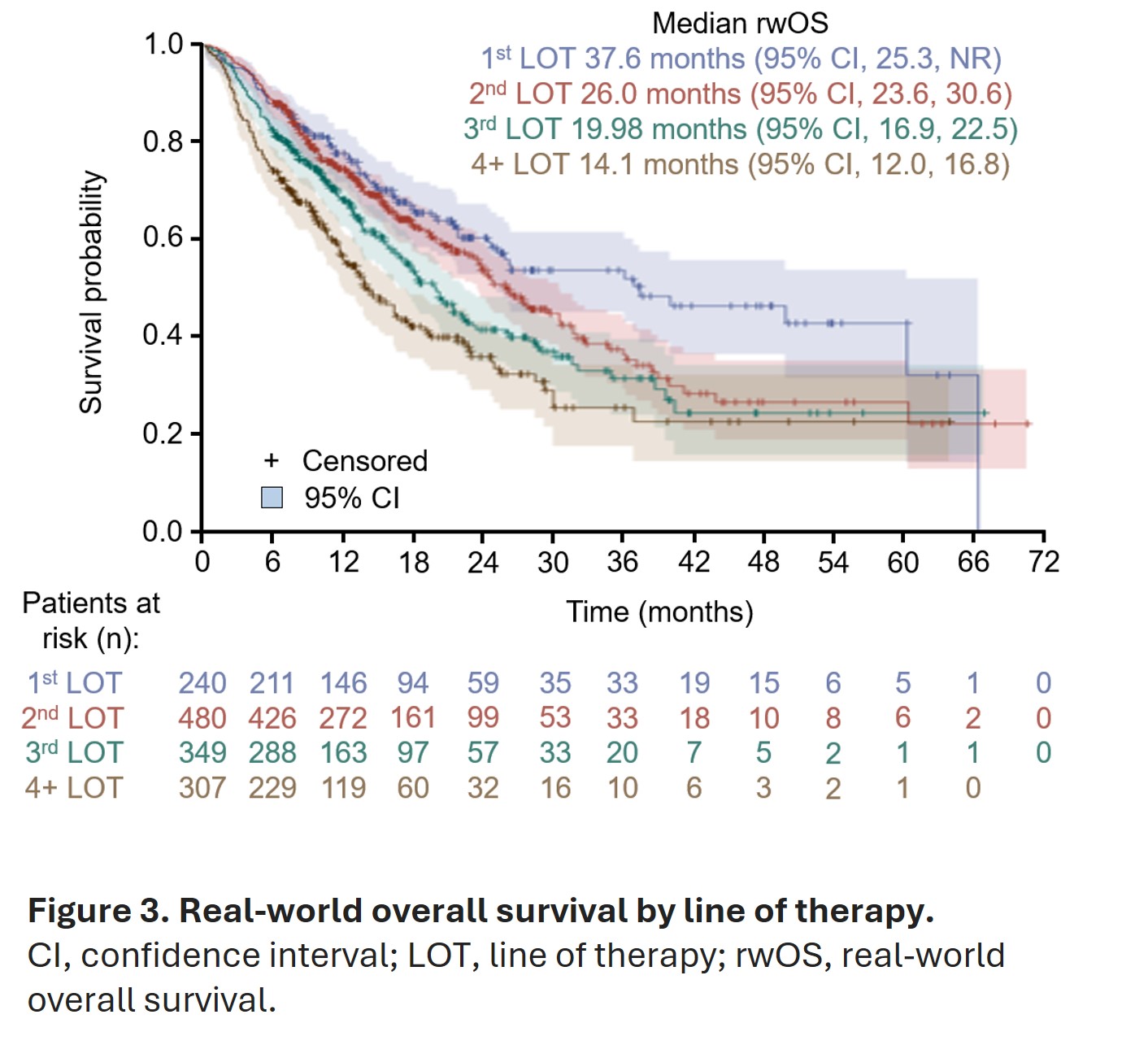
- Median real-world OS was longest in men who received radium-223 as their first line of therapy (37.6 months), and OS decreased incrementally in subsequent lines of therapy (Figure 3)
- When compared with radium-223 used as fourth line of therapy or later, use of radium-223 as first, second, or third line of therapy was associated with 58%, 42%, or 25% lower risk of death, respectively
What do these results mean?
This study – the largest real-world analysis of men treated with radium-223 in the US – supports the use of radium-223 as an earlier line of therapy for the treatment of mCRPC. The findings also support giving radium-223 in combination with other life-prolonging therapies, and highlight the importance of completing ≥5 cycles of treatment to improve overall survival. Clinicians may use this real-world evidence of radium-223 utilization and outcomes to guide future treatment decision making in patients with mCRPC.
Follow the Topic
-
Prostate Cancer and Prostatic Diseases

This journal covers all aspects of prostatic diseases, in particular prostate cancer, the subject of intensive basic and clinical research world-wide.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in