Reconstructing parasite development and the intracellular niche of a microsporidian parasite in 3D
Published in Cell & Molecular Biology

Microsporidia, an early-diverging group of fungi, are single-celled eukaryotic parasites that infect most animals including humans. Most commonly, human microsporidiosis manifests as an enteric infection, which is self-limiting in healthy individuals but can be fatal in immunocompromised patients. Human microsporidia infections are often caused by Encephalitozoon intestinalis, which initially invades and replicates within the mucosal epithelial lining of the small intestine. Like other intracellular parasites, microsporidia have evolved strategies to invade and co-exist with their hosts. One proposed invasion strategy involves an organelle unique to microsporidia called the polar tube that is coiled within the dormant spore. During infection, the polar tube is released from the spore and is thought to attach to a host cell, facilitating the transfer of infectious cargo from the spore to the host. Inside the host cell, E. intestinalis undergoes a developmental cycle within a parasitophorous vacuole.
Our understanding of how E. intestinalis organelles, in particular the polar tube, are assembled during development, remains rudimentary. Moreover, the physical basis for interactions of the parasitophorous vacuole with host organelles in the intracellular niche are not well understood. In this study we have used a volume EM technique called serial block-face scanning electron microscopy (SBF-SEM) to acquire a series of 2D images of infected cells, which are subsequently processed, annotated and reconstructed in 3D. Our 3D reconstructions of infected cells capture the parasites at different stages of development. The earliest stage of development captured in our datasets is the sporont stage in which only the nucleus and endoplasmic reticulum are discernable. The further development into sporoblasts and then spores is accompanied with the completion of cellularization and the development of a spore coat and other parasite organelles, such as the polar tube, posterior vacuole, anterior polaroplast and finally an anchoring disc.
Our 3D reconstructions captured different polar tube intermediates during parasite development and allowed us to propose a model for how the polar tube develops de novo (Figure 1A-F). In our model, development of the polar tube begins during the sporoblast stage, in the middle of the cell from a nucleation center. To build the linear segment of the polar tube, new protein subunits are added at the junction between the nucleation center and the polar tube. As the straight segment of the polar tube continues to grow, it likely encounters the bottom of the spore and begins to coil. Further polar tube growth results in an increase in the length of the polar tube and the number of coils. In dormant spores, the polar tube has 5-6 coils at the posterior end of the spore and is capped by the anchoring disc at the anterior end of the spore.
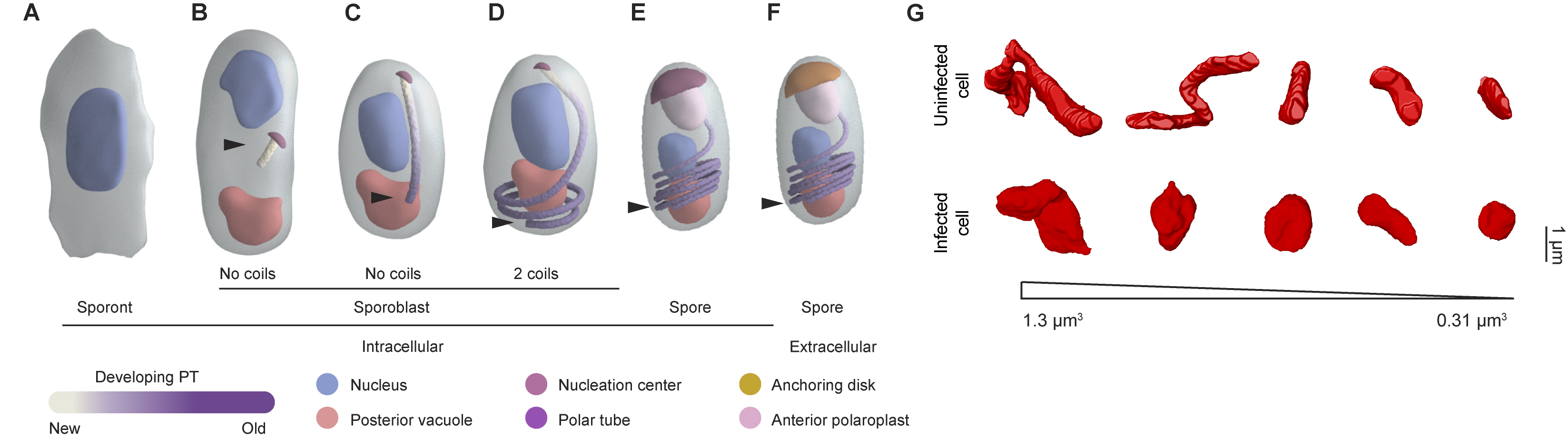
Figure 1: (A-F) Model of polar tube development. Arrowheads indicate the polar tube as it develops from early to late stages of E. intestinalis development. See Supplementary Movie 12 in Antao et al. 2023 for further information. (G) A comparison of mitochondria from uninfected and infected cells, showing the change in mitochondrial morphology observed.
3D reconstructions of the intracellular niche occupied by E. intestinalis revealed that the parasites develop in close proximity to the host mitochondria, consistent with previous observations. The host mitochondria were also substantially remodeled in infected cells, with the mitochondria being shorter and more spherical (Figure 1G). Live-cell imaging of the host mitochondrial network in infected cells revealed localized changes to the mitochondrial network around the parasitophorous vacuole, from more filamentous to fragmented mitochondria. As the parasite burden on the host cell increased, a global fragmentation of the host mitochondrial network was observed.
These findings build on previous work and allow us to propose models for the asynchronous development of microsporidia, the development of the polar tube, and the remodeling of host mitochondria by microsporidia.
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Your space to connect: The Myeloid cell function and dysfunction Hub
A new Communities’ space to connect, collaborate, and explore research on Clinical Medicine and Cell Biology!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in