Recovering injured lungs with cross-circulation
Published in Bioengineering & Biotechnology

Building an idea
No one likes waiting, especially when life hangs in the balance.
Today, tens of thousands of people are on a waiting list and are in desperate need of lung transplantation. But, unfortunately, there is a shortage of donor lungs, and because lungs are so susceptible to damage, only 1 out of 5 donated lungs can actually be used.
Inspired by the need to expand the pool of donor lungs, in 2012 our group set out to develop an organ rescue platform that enables the regeneration and recovery of injured donor lungs not initially usable for transplantation.
We started by developing ‘Deep Breaths,’ an ex vivo lung perfusion (EVLP) device capable of supporting lungs outside the body for a few hours. However, like other EVLP systems, Deep Breaths is an isolated circuit by design, disconnected from all the other organs, and therefore unable to provide lungs the critical systemic homeostatic regulation that is essential for longer term support.
Faced with the significant challenge of engineering a medical device that could provide the function of multiple organs like the liver and kidney, we took a page from the history book and pondered whether an abandoned surgical procedure called ‘cross-circulation’ – the exchange of blood flow between two patients – could be re-configured to help recover damaged donor organs.
The team – a key to success
In order to achieve cross-circulation between a living recipient and an extracorporeal organ, considerable technical expertise and innovation would be required. A special interdisciplinary collaboration led by Matthew Bacchetta, MD, MBA (lung transplant surgeon and director of clinical ECMO) and Gordana Vunjak-Novakovic, PhD (biomedical engineering professor) enabled an important physician-engineer partnership between Brandon Guenthart, MD (surgeon) and John O’Neill (biomedical engineer), the lead authors of the paper (Fig.1a).
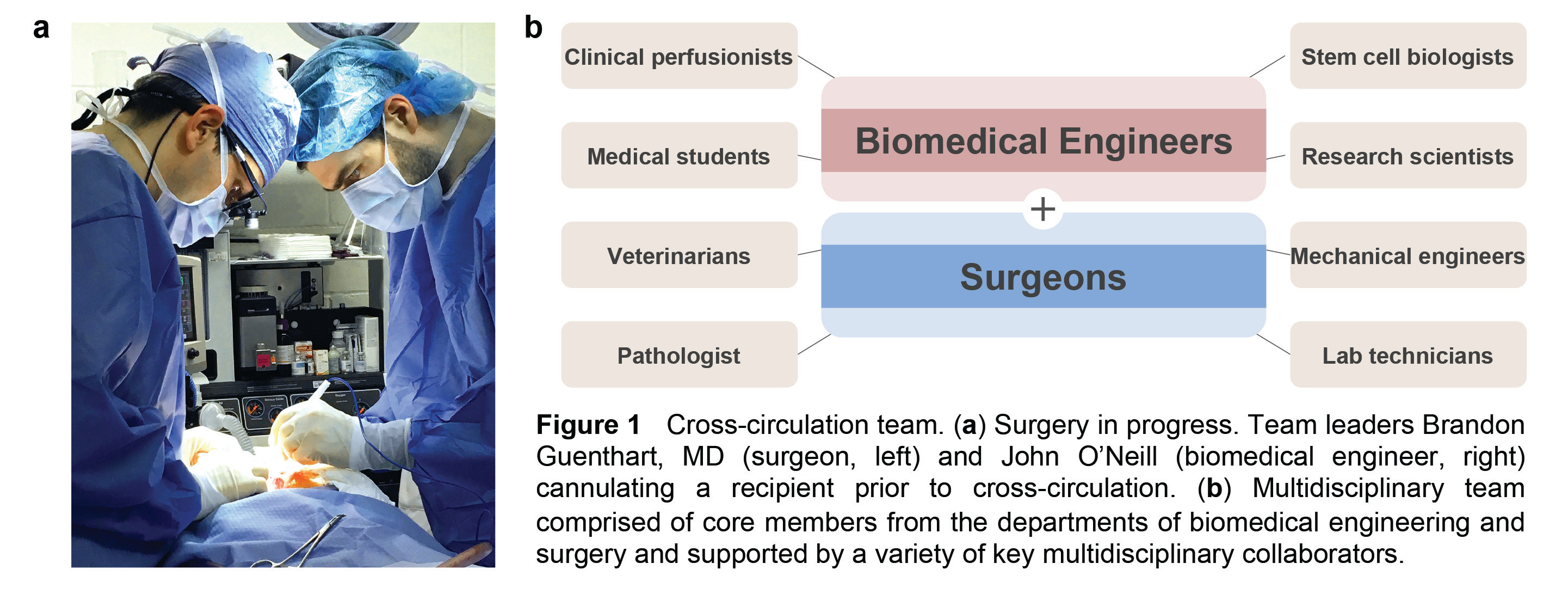
This close collaboration between surgeons and biomedical engineers melded two complementary skill sets, expanded our team’s resources, and attracted other key collaborators to the project. The surgical procedures and scientific analysis required coordinated teamwork and expertise across diverse areas of biomedical science and clinical care (Fig. 1b). Just imagine a busy operating room with all of the following going on: surgeons operating, veterinarians providing animal care, lab technicians analyzing samples, medical students recording data, research scientists performing biologic assays, stem cell biologists preparing therapeutic cells, bioengineers manipulating advanced imaging systems or measuring and adjusting physiologic parameters, and more!
Our main goal was to maximize clinical relevance while pioneering a novel procedure through rigorous scientific investigation and analysis. To accelerate translatability, we ensured that everything used during surgery and cross-circulation (besides a few customized components designed and manufactured in our lab) is already approved and in clinical use. Ultimately, we successfully achieved cross-circulation support of extracorporeal lungs and demonstrated recovery of injured lungs with this technique for the first time.
Overcoming challenges – innovation born out of necessity
As anyone who has conducted animal work is aware, there are many challenges before an experiment even begins. Our initial challenge was to convince skeptics that cross-circulation was an idea worth pursuing. Prior to our group, no one at our institution had attempted such long multi-day experiments, so questions regarding staffing, operating room utilization, and cost came to the forefront. Ultimately, after much discussion, we were granted approval to conduct a pilot study and run cross-circulation for 36 hours, with generous funding support from Columbia University, the NIH, and the Mikati Foundation.
As we developed our cross-circulation platform, we encountered additional challenges. To our knowledge, we were keeping viable lungs outside the body for 5-6 times longer than any platform ever had before. So, for example, to prevent the outer surface of the lung (the pleura) from drying out and to keep the lungs at body temperature, we developed a humidification system with ambient temperature control and a re-circulating warm water organ basin to mimic the thoracic cavity.
Next was the perfusion circuit. To allow for adequate blood flow into and out of the lungs during cross-circulation, we had to develop new cannulas and a new cannulation technique using a donor vessel as a “bio-bridge.” We also engineered a dynamic system capable of height and hydrostatic pressure adjustments, and feedback-regulated pressure-limited flow. As our work progressed, we continued to innovate out of necessity and streamline our cross-circulation setup and procedure. The videos below demonstrate reperfusion of extracorporeal lungs following procurement and the process of recruitment with adjustments to ventilation.
Video 1 | Reperfusion of extracorporeal lungs following lung procurement and flush.
Video 2 | Recruitment and expansion of collapsed extracorporeal lungs (2x speed).
Clinical impact – ongoing investigations
Cross-circulation has proven to be a valuable tool for investigation and has fostered interdisciplinary collaborations. In addition to our ongoing work in donor lung recovery and transplant models, our ability to keep lungs alive and functioning outside the body for days is giving us and other researchers new opportunities to investigate donor-recipient immunologic interactions, therapeutic cell delivery, stem cell differentiation, acute lung injury, and the development of new pulmonary theranostics. We also envision that cross-circulation could be applied to other transplantable organs, bioengineered grafts, and xenotransplantation models.
At our center, and around the world, patients in complete lung failure continue to wait and, unfortunately, many will die waiting. At the same time, surgeons are forced to decline donor lungs that fail to meet transplant criteria. Our hope is that this work will help address the shortage of donor lungs by enabling the recovery of life-saving organs and give dying patients a new chance at life.
Written by Brandon Guenthart, MD (bg2549@columbia.edu) and John O’Neill (jdo2116@columbia.edu).
Our paper: O’Neill, J. D. et al. Cross-circulation for extracorporeal support and recovery of the lung. Nat. Biomed. Eng. 1, 0037 (2017).




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in