RNA circles control neuronal activity and behavior
Published in Neuroscience and Genetics & Genomics
Schizophrenia (SCZ) and Bipolar Disorder (BD) combined affect 2-3.5 percent of the population1,2. The cognitive symptoms associated with these disorders, such as impaired behavioral flexibility and reward seeking, are highly disruptive to daily life and one’s ability to hold a job or maintain relationships3. Unfortunately, current therapeutics are not efficacious for many with these disorders3. With the recent expansion of genomics research, there has been a push towards precision medicine using molecular and genetic biomarkers to predict symptoms and disease progression. Understanding individual genetic risk can help inform therapeutic development targeted toward specific behavioral phenotypes and disease subtypes.
Circular RNAs (circRNAs), once thought to be transcriptional junk arising from splicing errors4, are stable biomolecules due to their lack of free ends that generally trigger degradation5. For this reason, they are becoming popular as biomarkers for diseases such as cancer6. Because circRNAs are stable and can be assayed from the blood, they offer a promising avenue for therapeutic development for complex diseases.
Our previous work determined circHomer1 to be downregulated in the postmortem fronto-cortical brain samples from patients with SCZ and BD7,8. circHomer1 levels were also downregulated in peripheral blood cells derived from BD patients7 and correlated with the age of SCZ onset8. In particular, BD patients with psychosis at the time of death showed the lowest expression of circHomer17, indicating circHomer1 levels could serve as a potential biomarker at least for a subset of patients experiencing severe disease.
circHomer1 is a highly conserved circRNA enriched in neurons8,9. Like its linear counterpart, Homer1a, circHomer1 is activity regulated9, meaning its expression increases following heightened neuronal activity. HOMER1 proteins are most well-known for their roles in homeostatic synaptic plasticity, which is a mechanism for maintaining synaptic firing probability to prevent runaway excitability that can lead to cell death10-12. Our previous work indicated circHomer1 influences the expression and synaptic localization of linear Homer1 transcripts and alters RNA expression of synaptic-associated genes7,8. We also demonstrated that targeted knockdown of circHomer1 within the fronto-cortical region of mice resulted in a specific executive function deficit marked by impaired reversal learning7,8.
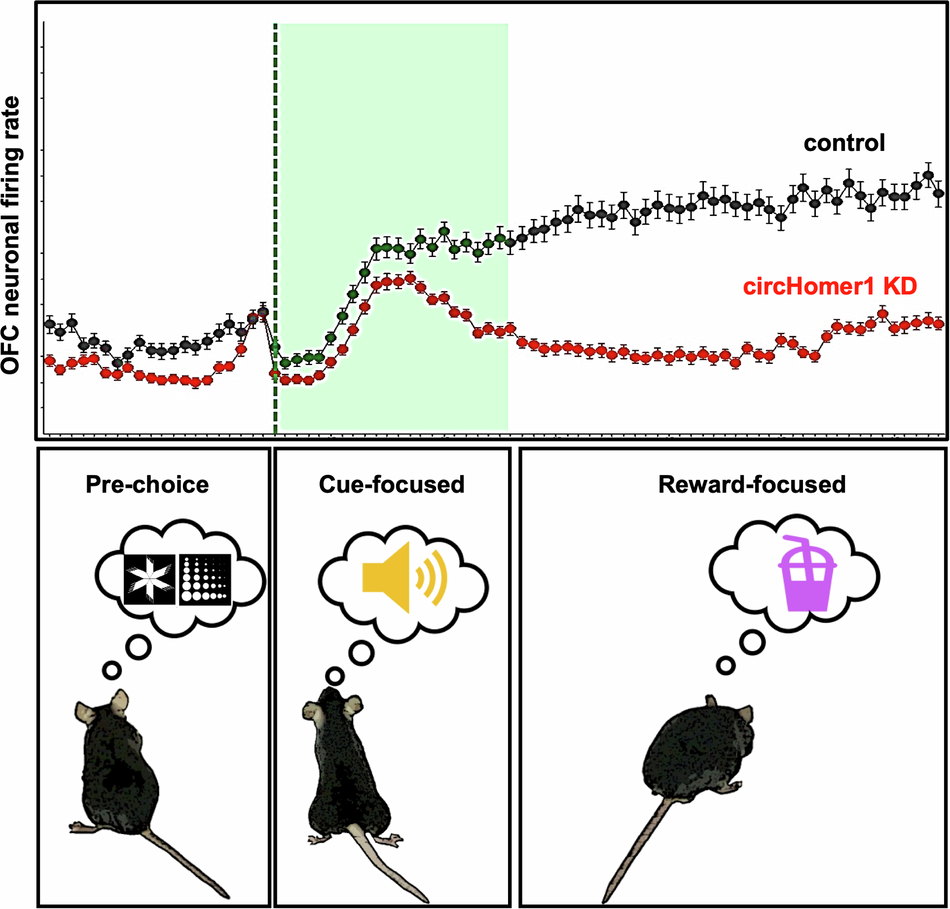
In the current study (https://doi.org/10.1038/s41398-024-03188-0), we hypothesized that loss of circHomer1 would alter neuronal firing activity during this reversal learning task leading to the impairment. To test this, we knocked down circHomer1 using a highly specific sh-RNA, which targets the unique splice junction of the mature circHomer1 product so as to not directly degrade the linear transcripts. We then implanted microarrays containing electrode wires spaced to target individual neuronal units within both hemispheres of the orbitofrontal cortex. The neuronal activity of mice was recorded during the reversal learning task, which utilizes a touchscreen that displays images associated with either a strawberry milk reward paired with a reinforcing tone or a punishment of a bright light paired with a trial timeout. circHomer1 knockdown animals showed neuronal firing changes that coincided with the behavioral deficit during the mid-stage reversal. The reversal learning task consists of multiple stages; initially mice must learn one shape is correct to a high degree (85% correct responses). Once they have established the correct choice, mice are faced with a reversal; now the previously rewarded shape is incorrect and the previous incorrect shape is now rewarded. This cognitive “reversal” requires the orbitofrontal cortex to recognize the change in reward contingencies and update one’s behavioral response. It is a function that is often disrupted in patients with psychiatric disorders and can best be thought of in the context of gambling or substance abuse where one must inhibit responding in the case an outcome is no longer rewarding. Interestingly, neurons from the circHomer1 knockdown animals had a reduced baseline firing rate and preferentially responded to the reinforcing tone rather than the reward during mid-stage reversal. We also found that the coordination of neuronal firing was mis-timed during learning. Specifically, the phase of neuronal synchronization immediately following the choice was prolonged throughout mid-stage reversal in knockdown animals. This heightened neuronal phase coherence is generally associated with a novel, salient event that requires attention and should diminish as a task is learned. The knockdown animals, however, appeared to continue to allocate attention to the choice for longer, increasing the time it took for them to effectively learn the new association. In normal animals, we would expect that coordinated neuronal activity that is appropriately timed to a choice would result in a change to the amplitude or strength of the neuronal signal (i.e. the neuronal power) relative to the baseline signal. However, the neuronal power fluctuations following a choice were largely diminished in circHomer1 knockdown animals during mid-stage reversal indicating reduced neuronal recruitment toward the task. We did, indeed, observe a reduction in the proportion of correct choice-responsive neurons during mid-to-late reversal learning, which likely underlies this observation. Together, it appears the loss of circHomer1 reduces neuronal network activity, and to compensate neurons become hypersynchronous.
To determine the molecular mechanism underlying this blunted signaling response, we measured RNA from knockdown animals and found a reduction of all measured synaptic-associated genes. Given these mice had a stable loss of circHomer1 for multiple months, these RNA changes likely reflect a global downscaling of synaptic-associated genes in an attempt to balance neuronal network activity demands.
circHomer1 downregulation has been observed in a variety of psychiatric and neurocognitive disorders. As circHomer1 has been found to be activity regulated and is known to play a role in synaptic gene regulation, we hypothesize that circHomer1 is acting similar to Homer1a to influence synaptic scaffold rearrangement in response to network demands. In disorders such as SCZ and BD, there is an improper maintenance of homeostatic plasticity. Certain antipsychotics and mood stabilizers such as lithium and ketamine act to influence homeostatic synaptic plasticity. Since circHomer1 expression is associated with neural correlates of behavior as well as molecular signatures of synaptic downscaling, it could serve as a biomarker of brain state and inform potential therapeutic response.
- McGrath, J., Saha, S., Chant, D., and Welham, J. (2008). Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev 30, 67-76. 10.1093/epirev/mxn001.
- Merikangas, K.R., He, J.P., Burstein, M., Swanson, S.A., Avenevoli, S., Cui, L., Benjet, C., Georgiades, K., and Swendsen, J. (2010). Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 49, 980-989. 10.1016/j.jaac.2010.05.017.
- Patel, K.R., Cherian, J., Gohil, K., and Atkinson, D. (2014). Schizophrenia: overview and treatment options. P T 39, 638-645.
- Cocquerelle, C., Mascrez, B., Hetuin, D., and Bailleul, B. (1993). Mis-splicing yields circular RNA molecules. FASEB J 7, 155-160. 10.1096/fasebj.7.1.7678559.
- Cortes-Lopez, M., and Miura, P. (2016). Emerging Functions of Circular RNAs. Yale J Biol Med 89, 527-537.
- Conn, V.M., Chinnaiyan, A.M., and Conn, S.J. (2024). Circular RNA in cancer. Nat Rev Cancer 24, 597-613. 10.1038/s41568-024-00721-7.
- Hafez, A.K., Zimmerman, A.J., Papageorgiou, G., Chandrasekaran, J., Amoah, S.K., Lin, R., Lozano, E., Pierotti, C., Dell'Orco, M., Hartley, B.J., et al. (2022). A bidirectional competitive interaction between circHomer1 and Homer1b within the orbitofrontal cortex regulates reversal learning. Cell Rep 38, 110282. 10.1016/j.celrep.2021.110282.
- Zimmerman, A.J., Hafez, A.K., Amoah, S.K., Rodriguez, B.A., Dell'Orco, M., Lozano, E., Hartley, B.J., Alural, B., Lalonde, J., Chander, P., et al. (2020). A psychiatric disease-related circular RNA controls synaptic gene expression and cognition. Mol Psychiatry 25, 2712-2727. 10.1038/s41380-020-0653-4.
- You, X., Vlatkovic, I., Babic, A., Will, T., Epstein, I., Tushev, G., Akbalik, G., Wang, M., Glock, C., Quedenau, C., et al. (2015). Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci 18, 603-610. 10.1038/nn.3975.
- Clifton, N.E., Trent, S., Thomas, K.L., and Hall, J. (2019). Regulation and Function of Activity-Dependent Homer in Synaptic Plasticity. Mol Neuropsychiatry 5, 147-161. 10.1159/000500267.
- Hu, J.H., Park, J.M., Park, S., Xiao, B., Dehoff, M.H., Kim, S., Hayashi, T., Schwarz, M.K., Huganir, R.L., Seeburg, P.H., et al. (2010). Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron 68, 1128-1142. 10.1016/j.neuron.2010.11.008.
- Wang, Y., Rao, W., Zhang, C., Zhang, C., Liu, M.D., Han, F., Yao, L.B., Han, H., Luo, P., Su, N., and Fei, Z. (2015). Scaffolding protein Homer1a protects against NMDA-induced neuronal injury. Cell Death Dis 6, e1843. 10.1038/cddis.2015.216.
Follow the Topic
-
Translational Psychiatry

This journal focuses on papers that directly study psychiatric disorders and bring new discovery into clinical practice.
Related Collections
With Collections, you can get published faster and increase your visibility.
Moving towards mechanism, causality and novel therapeutic interventions in translational psychiatry: focus on the microbiome-gut-brain axis
Publishing Model: Open Access
Deadline: May 19, 2026
From mechanism to intervention: translational psychiatry of childhood maltreatment
Publishing Model: Open Access
Deadline: Jun 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in