Role of formaldehyde in promoting aromatic selectivity during methanol conversion over gallium-modified zeolites
Published in Chemistry

Aromatics, especially benzene, toluene, and xylenes (BTX), are key building blocks in the petrochemical industry. The production of aromatics strongly lies in a fossil-based process such as catalytic reforming and naphtha cracking. The dilemma on the upsurging demands of BTX against the increasing depletion of oil reserves urged us to develop some new nonpetroleum routes to produce these chemicals. Over the past decades, different processes, like the aromatization of alkenes and alkanes, biomass pyrolysis, and methanol to aromatics (MTA), have been paid much efforts to increase in aromatic selectivity. Among them, the MTA process as an alternative to produce BTX attracts extensive attention, because methanol can be easily obtained from a wide source such as coal, natural gas and biomass.1
Gallium-modified HZSM-5 zeolites are known to increase aromatic selectivity in methanol conversion. For Ga-modified HZSM-5, both cationic metal species (Ga3+) and/or metal oxides (Ga2O3) have been reported to result in a significant enhancement in aromatic production. Previous works suggested that cationic Ga species are formed as Lewis acid sites by substitution of Brønsted acid sites on H-ZSM-5 modified by Ga metal via impregnation or ion exchange.2 The cationic Ga species cooperating with BASs facilitates the dehydrogenation-aromatization processes of cycloalkenes, which is called the LAS-induced aromatic formation pathway. The significant increase in the amount of H2 production matched well with the trend of aromatics formation, confirming the prevailing dehydrogenative process on Ga/HZSM-5 catalysts.3 The enhanced selectivity to aromatics over the composites of Ga2O3 clusters and zeolite ZSM-5 was attributed to the Ga2O3/zeolite interface region which displayed enhanced dehydrogenation properties. 4
In this work, we studied methanol conversion over Ga-modified HZSM-5 using in situ synchrotron radiation photoionization mass spectrometry. To capture the active intermediates during methanol conversion, a 2 Torr pressure was applied to the catalytic reactor, under which gas collision was reduced so that the lifetime of intermediates was prolonged. We found that formaldehyde (HCHO) was remarkably boosted over Ga-impregnated HZSM-5, along with the enhanced selectivity to aromatics. After eliminating HCHO into CO and H2 by physically mixing Y2O3 with Ga-impregnated HZSM-5, aromatic production decreased to the level of parent HZSM-5. It indicated that there existed a new pathway to generate aromatics involving formaldehyde participation, which is called as HCHO-induced aromatic formation pathway. Recently, HCHO has aroused wide interest from many investigators, because of its important role in forming first carbon-carbon bond, chain propagation, and aromatics formation. Lercher et al. suggested that HCHO can react with olefins to form dienes via Prins reaction (HCHO + olefins → dienes + H2O), and dienes react stepwise with HCHO to form H-poor products, aromatics and eventually cokes.5
After that, the formation mechanism of extra HCHO and the involved active sites in Ga-modified HZSM-5 were systematically studied. We verified that the direct dehydrogenation of methanol over Ga2O3 clusters contributed the more HCHO production. Cationic GaO+ species had no activity to generate HCHO but promoted the dehydrogenative aromatization process of alkenes. In addition, an interesting phenomenon was observed in which the HCHO yield sharply increased after reduction-oxidation treatment of Ga-impregnated HZSM-5. It can be attributed to the migration of gallium from the external surface of the zeolite crystallites to their intracrystalline volume after reduction-oxidation treatment, leading to higher cationic GaO+ concentration and redispersed Ga2O3 (Figure 1). Thus, both the LAS-induced and HCHO-induced aromatic formation pathways were enhanced, resulting in higher aromatic selectivity and faster deactivation. We believe that the above insights into the role of formaldehyde in promoting aromatic selectivity during methanol conversion over gallium-modified zeolites will provide a new thought to manipulate the product distribution in the MTA reaction.
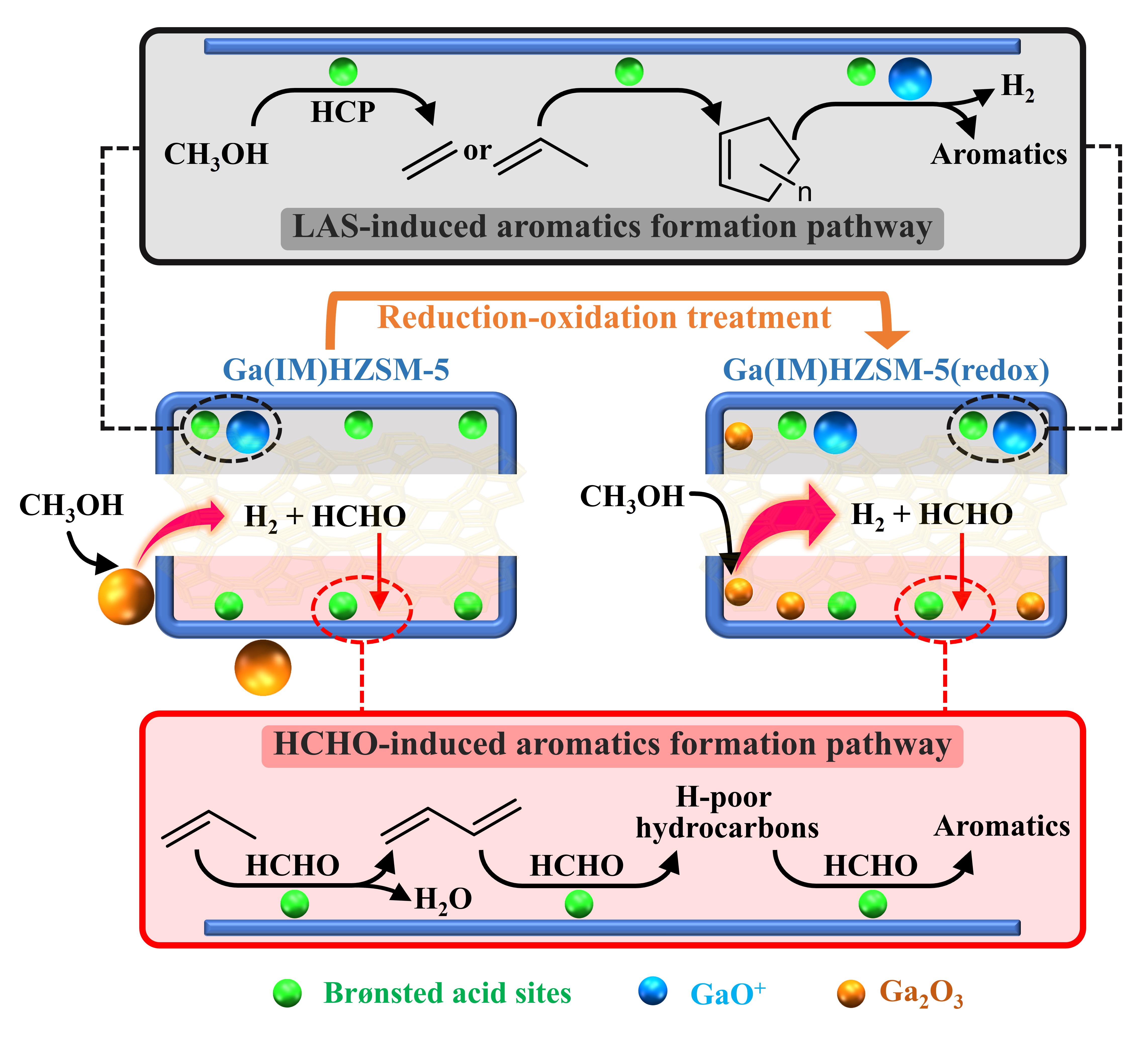
Figure 1. Proposed evolution mechanisms of catalyst and aromatic formation. Mechanistic pathways for the formation of HCHO on Ga2O3, migration and transformation of Ga species upon reduction‒oxidation treatment, and the two kinds of aromatic formation pathways.
References
1 Olah, G. A. Beyond oil and gas: The methanol economy. Angew. Chem. Int. Ed. Engl. 44, 2636-2639, (2005).
2 Gao, P. et al. Bronsted/Lewis acid synergy in methanol-to-aromatics conversion on Ga-modified ZSM-5 zeolites, as studied by solid-state NMR spectroscopy. ACS Catal. 8, 69-74, (2018).
3 Gao, P. et al. A mechanistic study of methanol-to-aromatics reaction over Ga-modified ZSM-5 zeolites: understanding the dehydrogenation process. ACS Catal. 8, 9809-9820, (2018).
4 Lopez-Sanchez, J. A. et al. Reactivity of Ga2O3 clusters on zeolite ZSM-5 for the conversion of methanol to aromatics. Catal. Lett. 142, 1049-1056, (2012).
5 Muller, S. et al. Hydrogen transfer pathways during zeolite catalyzed methanol conversion to hydrocarbons. J. Am. Chem. Soc. 138, 15994-16003, (2016).
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026
Advances in Asymmetric Catalysis for Organic Chemistry
Publishing Model: Open Access
Deadline: Mar 31, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in