Understanding proton transport is an old and fundamental problem critical for many technologies, including energy generation, storage, and conversion along with many bio-chemical processes. Recently, it has become even more important due to the return in interest in a Hydrogen Economy and Blue Energy.
It is generally understood that proton transport occurs via two primary mechanisms: 1) the vehicular mechanism, where the proton moves along with a host molecule (Fig. 1a), and 2) the hopping or shuttling mechanism, which involves proton transfers between different host molecules through elementary proton jumps (Fig. 1b). However, the proton hopping mechanism can be collective with correlated proton jumps in the same direction along hydrogen-bonded chains of host molecules. This type of collective hopping facilitates an efficient transport of charge (Fig. 1c). This idea of correlated proton transport was initially proposed by Grotthuss more than 200 years ago. Although the Grotthuss mechanism appears simple, and many computational studies have suggested its existence, experimental verification has remained a challenge.
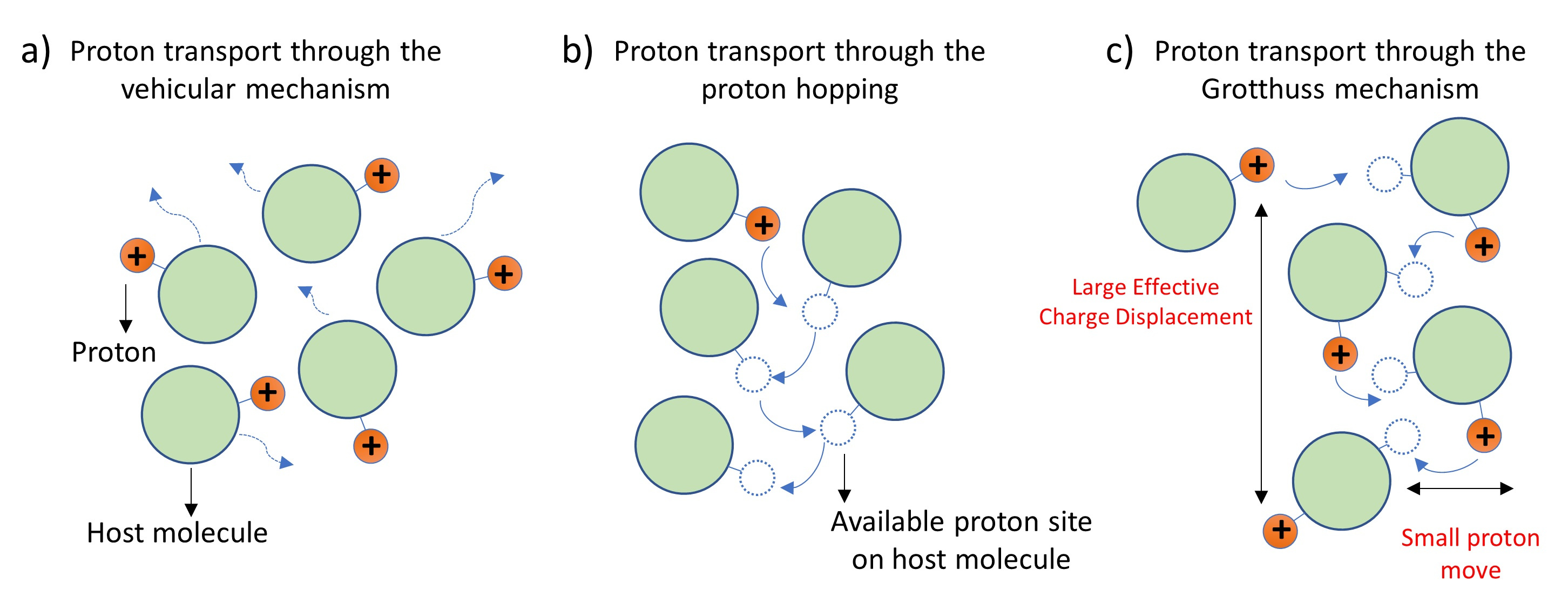
Figure 1. Different mechanisms for proton transport: a) Vehicular mechanism; b) Proton hopping; c) Grotthuss mechanism.
To address this historical challenge, researchers from the University of Tennessee and Oak Ridge National Laboratory employed a combination of advanced experimental techniques and ab initio molecular dynamic (AIMD) simulations to study the fast proton dynamics in phosphoric acid and its aqueous solution [1]. These systems are known to be the best intrinsic proton conductors. Experimental techniques included: 1) high frequency dielectric measurements to detect the characteristic timescale of proton transport; 2) quasielastic neutron scattering to directly measure proton jump length and time, as well as the number of protons participating in hopping mechanism; 3) light scattering to measure the timescale of the molecular motion. The results of this comprehensive study show: i) all protons participate in the hopping process in these liquids; ii) the averaged distance for a proton jump is surprisingly short ~ 0.5-0.7 Å (in comparison, a typical ion jump length in ionic liquids is ~1-2 Å); and iii) the characteristic time of proton jumps is very short, much faster than molecular motions, and coincides with the conductivity timescale measured by broadband dielectric spectroscopy. The latter revealed that these fast and short proton jumps present the major mechanism of proton conductivity in phosphoric acid. AIMD simulations revealed that in most cases the proton indeed jumps very short distances of ~0.5 Å, while there are also some much longer jumps associated with the torsional rotation of the covalent OH bond around the PO bond axis (Fig. 2).
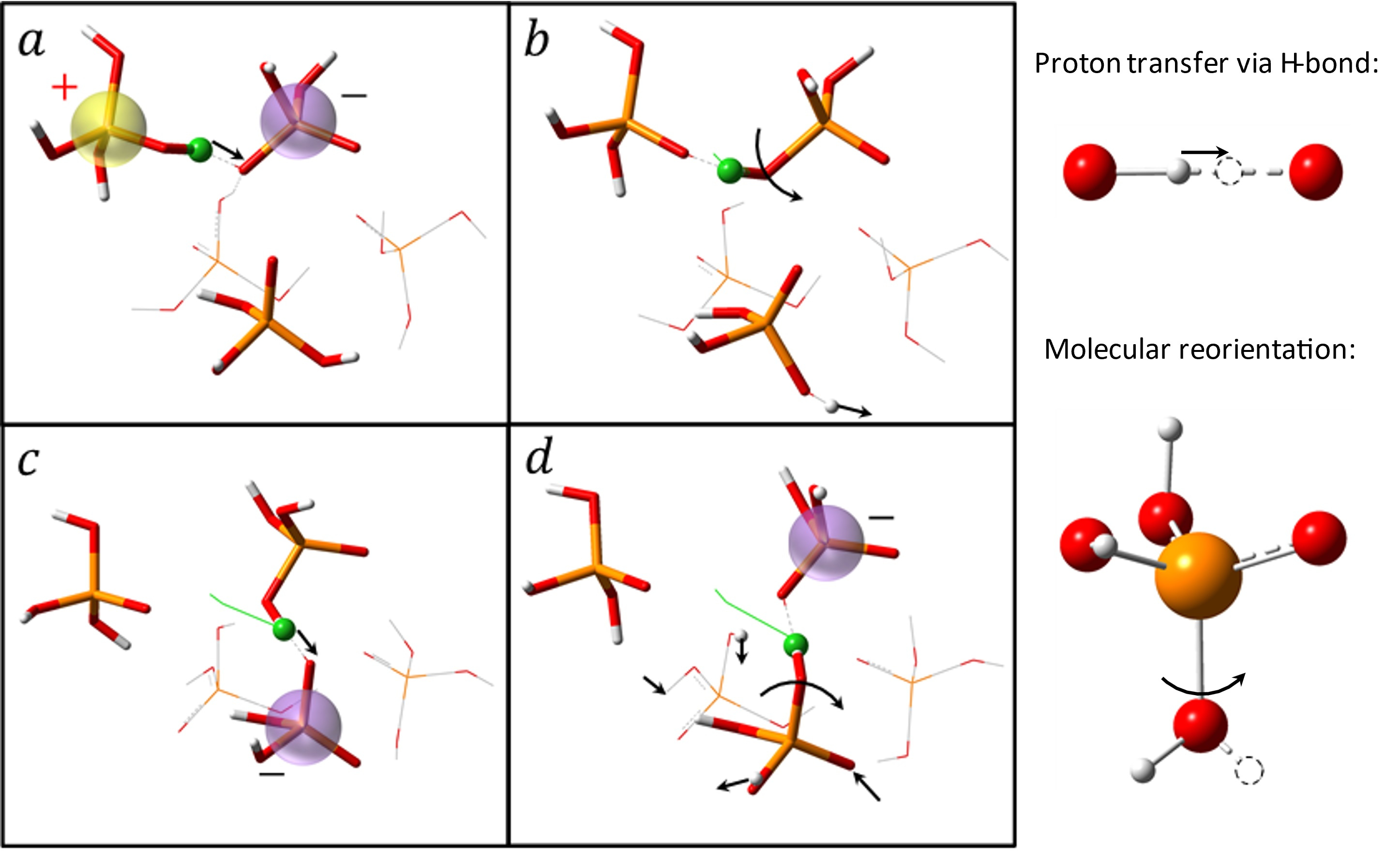
Figure 2. Snapshots of the elementary steps for proton transport in pure PA. The green ball represents the diffusive proton (white, hydrogen; red, oxygen; orange, phosphorus). The green lines show the trajectory line of the proton. The arrows denote the associated proton transfers and the reorientations of the covalent OH bonds around the PO bond axis in the mechanism. The charged entities were highlighted. The collaboration of proton transfers via the hydrogen bond (a and c) and large angular jumps (b and d) facilitates the long-range diffusion of protons.
Thus, the efficient and fast proton transport in phosphoric acid seems to be the best candidate for demonstrating the Grotthuss mechanism. To reveal this connection, we analyzed a parameter called the inverse Haven ratio, H-1, or ionicity. This parameter represents a simple ratio between the experimentally measured conductivity and the conductivity estimated from proton diffusivity and concentration. In the Grotthuss mechanism, the expected collective chain-like proton transfers should result in the proton conductivity being higher than that estimated from the proton diffusion, i.e. H−1>1. However, the analysis reveals that H−1<1, indicating that although proton jumps are correlated (collective), these correlations actually suppress conductivity instead of enhancing it, as expected in the Grotthuss mechanism. As a hypothesis, we suggested that Grotthuss chain-like proton conductivity may be impossible in bulk liquids. Possibly, the Grotthuss mechanism can only be realized in the presence of one-dimensional conducting channels, e.g., those found in some solid-state materials. Indeed, ionic conductivity in many superionic ceramics appears to be much faster than expected from the ion diffusion, with these materials exhibiting H−1>1.
- Ivan Popov, Zhenghao Zhu, Amanda R. Young-Gonzales, Robert L. Sacci, Eugene Mamontov, Catalin Gainaru, Stephen J. Paddison, Alexei P. Sokolov. Search for a Grotthuss mechanism through the observation of proton transfer. Communication Chemistry, 6, 77 (2023). DOI: https://doi.org/10.1038/s42004-023-00878-6
Follow the Topic
-
Communications Chemistry

An open access journal from Nature Portfolio publishing high-quality research, reviews and commentary in all areas of the chemical sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
f-block chemistry
Publishing Model: Open Access
Deadline: Feb 28, 2026
Experimental and computational methodology in structural biology
Publishing Model: Open Access
Deadline: Apr 30, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in