Secondary versus de novo ontogeny for AML in the era of molecular genetics
Published in Cancer
Background:
Acute myeloid leukemia (AML) is an aggressive blood cancer that can develop from a known antecedent hematologic disease (AHD), most often myelodysplastic syndrome (MDS), or due to prior chemotherapy or radiation given for a prior malignancy. When AML develops in these settings, it is considered secondary (sAML), either after AHD (post-AHD sAML) or therapy-related (tAML), respectively. In the absence of these historical features, AML is classified as “de novo” (dnAML), which is classically considered to have a better prognosis than sAML. However, multiple studies have indicated patients with specific genetic features have similar outcomes regardless of disease ontogeny.1,2 Additionally, specific genetic features segregate within rigorously-defined dnAML and sAML patient groups;3 accordingly, secondary-like mutations were notably incorporated into the European LeukemiaNet (ELN) prognostication scheme last year as adverse risk features.4 Collectively, these studies suggest that cytogenetic and molecular genetic features—as opposed to disease ontogeny—are responsible for the differing outcomes between dnAML and sAML. Further, often patients may be assumed to have secondary AML due to abnormal antecedent blood counts (ABCs), which could indicate previously undiagnosed AHD or be indicative of any number of non-hematologic comorbidities. In fact, the late Eli Estey, who is co-last author on this manuscript, would often view AML patients with abnormal ABCs as sAML even in the absence of proven AHD, and his categorization of patients inspired us to examine whether abnormal ABCs in dnAML patients were indicative of outcomes like those with known sAML. Additionally, we sought to evaluate whether ontogeny was prognostically significant after accounting for genetic risk as assessed by the ELN 2022 prognostication schema and TP53 mutation status.
We evaluated 734 patients with newly diagnosed AML from two academic medical centers (Johns Hopkins Hospital, Baltimore, MD and Massachusetts General Hospital, Boston, MA) for associations between ABC abnormalities, genetic risk, and outcomes. Patients with dnAML were evaluated for abnormal ABCs starting 28 days prior to their diagnosis and up to two years before diagnosis (Figure 1). ABCs were considered abnormal if there outside of the lab reference range for white blood cell count (excluding due to lymphocytosis), hemoglobin level, platelet count, absolute neutrophil count, absolute monocyte count, or absolute eosinophil count. Post-AHD sAML cases were defined by prior confirmed pathologic diagnosis of AHD, such as MDS or MDS/myeloproliferative neoplasm overlap. Genetic risk was assessed by ELN 2022 and TP53 mutation status. Outcomes included overall and relapse free survival (OS and RFS).
Main Findings:
We found patients with post-AHD sAML or tAML had worse survival than dnAML patients, as expected (Figure 2A). Interestingly, dnAML patients with abnormal ABCs had worse outcomes than those with normal ABCs (Figure 2B), but multivariate analysis demonstrated patients with dnAML and abnormal ABCs did not have a significantly increased risk of death compared to those with normal ABCs. Subsequently, we grouped all dnAML patients together regardless of ABC abnormalities to assess the impact of ontogeny. Post-AHD sAML and tAML no longer conferred a significantly increased hazard of death compared to dnAML after accounting for genetic factors. Finally, TP53 mutation status was associated with significantly increased hazard of death on multivariate analysis, even after adjusting for ELN 2022 adverse risk.
Conclusions and Next Steps:
Ultimately, our study suggests that abnormal ABCs do not have an impact on outcomes independent of disease genetics and other relevant covariates. Additionally, the risk attributed to ontogeny appears be accounted for by disease genetics, as measured by ELN 2022 and TP53 mutation. Many clinical trials use ontogeny (i.e., sAML vs dnAML) to determine study eligibility criteria, but these findings emphasize the value of using disease genetics instead. A practical limiting factor is the turnaround time of large NGS panels in providing the relevant information, but limited trial panels and advancing technology in this space may help push things forward in this regard. Finally, the unique adversity of TP53 mutations suggests that it may distinguish a prognostic group within AML even worse than that of the ELN 2022 adverse risk group, as suggested by others.5
References:
1 Othman J, Meggendorfer M, Tiacci E, Thiede C, Schlenk R, Dillon R et al. Overlapping features of therapy-related and de novo NPM1-mutated AML. Blood 2023; 141: 1846–1857.
2 Weinberg OK, Siddon A, Madanat YF, Gagan J, Arber DA, Dal Cin P et al. TP53 mutation defines a unique subgroup within complex karyotype de novo and therapy-related MDS/AML. Blood Adv 2022; 6: 2847–2853.
3 Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015; 125: 1367–1376.
4 Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022; 140: 1345–1377.
5 Rausch C, Rothenberg-Thurley M, Dufour A, Schneider S, Gittinger H, Sauerland C et al. Validation and refinement of the 2022 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia 2023; 37: 1234–1244.
Figures
Figure 1. Patients were assessed for abnormal antecedent blood counts from 28 days prior to dnAML diagnosis to up to two years (730 days) prior.
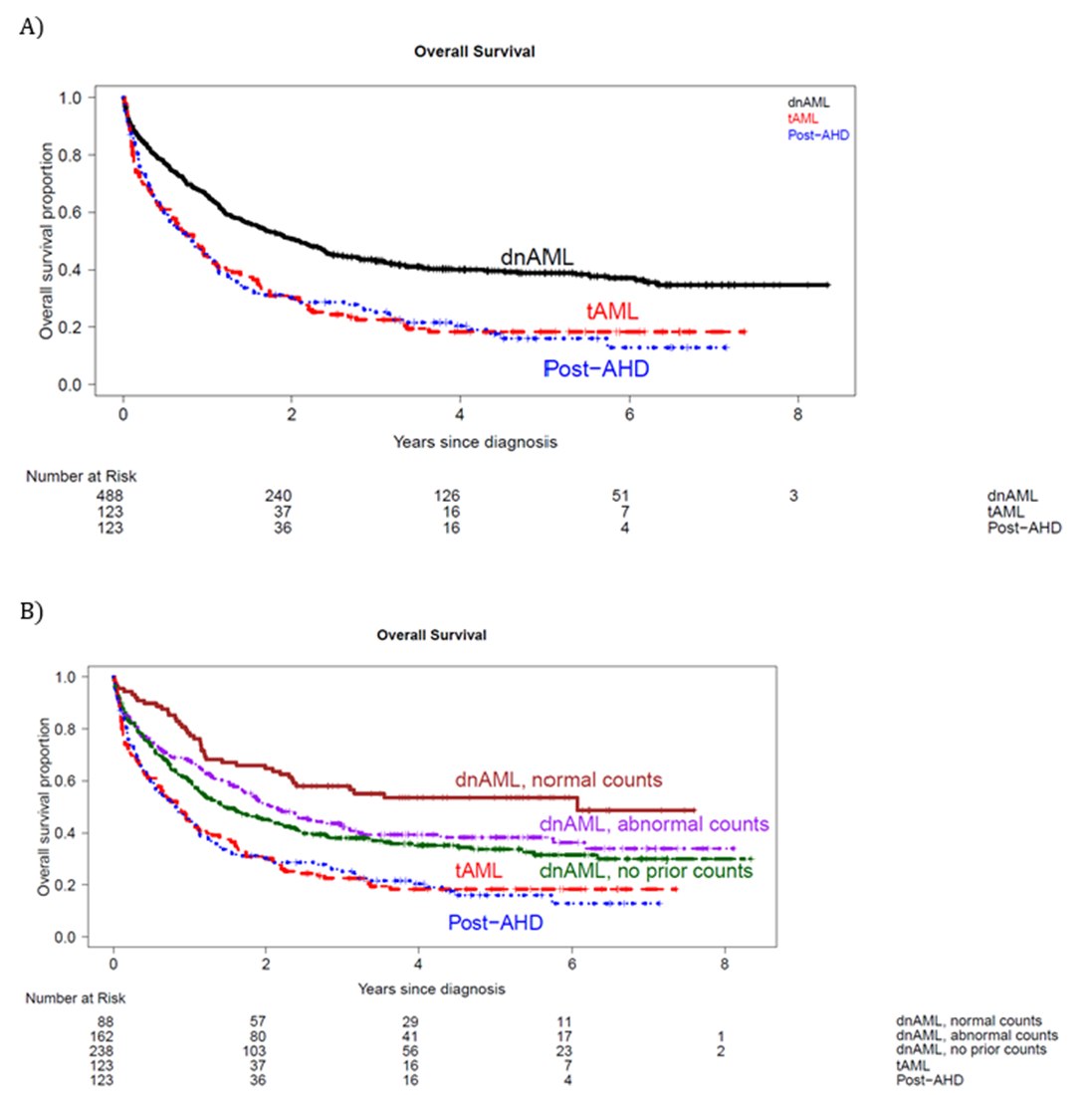
Figure 2. Overall survival of AML patients by A) ontogeny and B) ontogeny and antecedent blood counts for dnAML cases.
Follow the Topic
-
Leukemia

This journal publishes high quality, peer reviewed research that covers all aspects of the research and treatment of leukemia and allied diseases. Topics of interest include oncogenes, growth factors, stem cells, leukemia genomics, cell cycle, signal transduction and molecular targets for therapy.





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in