Seed bacteria are the architects of the complex wheat rhizosphere microbiome
Published in Ecology & Evolution, Microbiology, and Plant Science

Plants are more than just individual organisms—they are intricate ecosystems shaped by interactions with diverse microbial communities that influence their health, growth, and resilience. Beneath the soil, plant roots extend in search of water and nutrients, while also releasing carbon-rich molecules that attract diverse communities of bacteria, fungi and other microbes. These root-associated communities—collectively known as the rhizosphere microbiome—play a fundamental role in plant health and productivity, helping plants acquire nutrients, resist disease and pests (Harmsen et al., 2024), and withstand environmental stress. Therefore, these microbiomes impact the overall yield of crop plants and manipulating them holds promise for increasing crop productivity without relying on harmful chemical pesticides and herbicides.
For years, the soil microbiome was thought to be the primary driver of the rhizosphere community assembly. Since soils are a reservoir of microbial diversity and plants extend their roots into it, it seemed logical that roots would be colonized primarily by soil-dwelling bacteria. But in our recent research, we uncovered a different story—one where seed-borne microbes, inherited from previous plant generations, play an unexpectedly dominant role and act as architects of the wheat rhizosphere microbiome.

Rhizospheres of two wheat plants sampled in the Grandcour region, Switzerland. Photographed by Christoph Keel.
The question: How do root microbiomes form?
Wheat is one of the world’s most important crops, feeding billions of people. As with all plants, its roots are surrounded by a dense microbial ecosystem that influences wheat growth and health, yet we still don’t fully understand how its microbiome forms. Which microbes establish first, why, and do they assemble in a predictable way? One of the biggest challenges in studying root-associated microbiomes is their enormous complexity, which limits the extent of experimental testing. While simplified models allow researchers to reduce microbial diversity for controlled experiments, obtaining a reproducible yet complex plant microbiome remains a fundamental asset for testing hypotheses about microbiome assembly and manipulation.
These questions are critical because if we understand microbiome assembly, we can manipulate it to benefit crops. The ability to steer microbial communities towards beneficial configurations (Burz et al., 2023)—favoring microbes that promote plant health, suppress disease, or promote stress tolerance, could reduce the need for chemical pesticides and fertilizers. But before we can engineer microbiomes, we need to answer a fundamental question: where do root-associated microbiomes actually originate?
The experiment: Mimicking microbiome succession
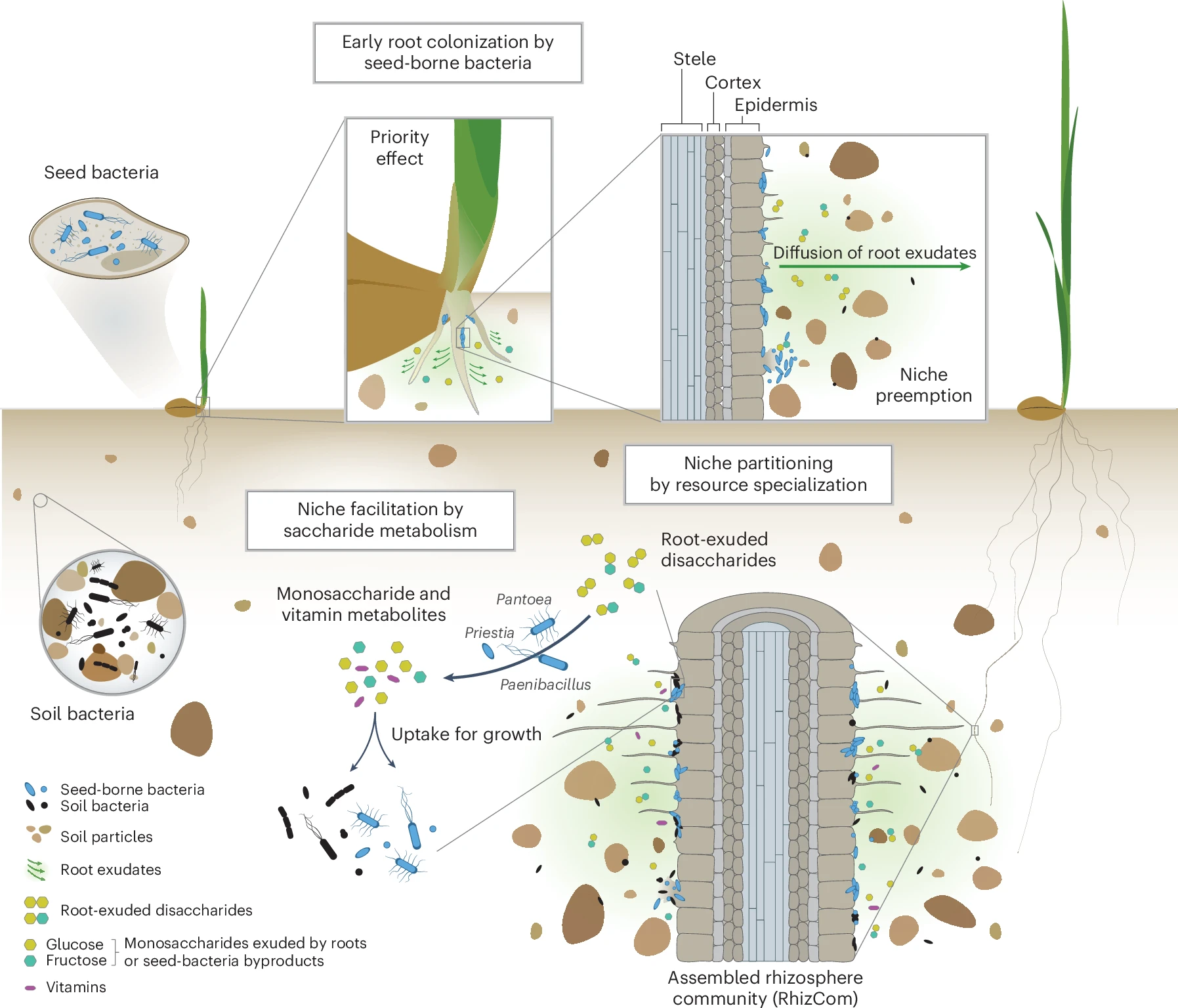
To address this question, we designed an experiment using sequential microbiome propagation—a method that mimics how microbial communities naturally evolve over time. First, we allowed the soil and seed-borne microbiomes to coalesce into a rhizosphere microbiome during the first plant growth cycle, allowing both sources to contribute to community assembly. Rather than starting fresh with each planting, we then transferred this established rhizosphere microbiome to the next generation of wheat, repeating the process over multiple cycles. This approach allowed us to test whether microbiomes assemble in a predictable and reproducible manner, and to determine which microbes exert the strongest influence on community assembly. By propagating the rhizosphere microbiome over generations, we aimed to stabilize the community and reveal the ecological principles governing its assembly.
Seed-borne vs. soil microbes: A skewed balance
Our most surprising finding was that bacteria carried to the next generation via seeds consistently outcompeted the native soil microbes to become the dominant members of the rhizosphere microbiome. This challenges the long-held assumption that soil microbes, owing to their sheer abundance and diversity, are the primary colonizers of plant roots. Instead, seed microbes arrive first and establish themselves early, shaping the entire trajectory of microbiome assembly.
We believe that the head start of seed-borne bacteria to colonize the rhizosphere first, further determines which microbes can establish later, a well-known ecological principle called the priority effect. By occupying key niches early, seed-borne bacteria could restrict soil microbes from taking hold, ultimately shaping the microbial landscape of the wheat rhizosphere.
Our findings also highlight a critical oversight in plant microbiome research: seed-associated bacteria are often neglected in many studies. This is largely due to the widespread assumption that soil bacteria dominate plant-microbe interactions, coupled with a lack of appropriate experimental controls to assess the influence of seed microbiota. Our results suggest that re-evaluating the role of seed-borne microbes is essential to fully understand plant-microbiome dynamics and to design effective microbiome-based agricultural strategies.
The key to success: Saccharide metabolism and niche partitioning
So why do seed-borne bacteria dominate? To investigate this, we used metagenomic sequencing, a technique that allows us to recover and analyze all the genetic material present in a microbial community at once, rather than isolating individual species. By sequencing and comparing the genetic content of soil, seed-borne, and rhizosphere microbiomes at an unprecedented depth, we identified key genetic differences that explain why seed-borne bacteria had an advantage and dominated the rhizosphere microbiome.
We found that both soil- and seed-borne bacteria had a similar genetic capacity to utilize simple sugars (monosaccharides) exuded by plant roots, which serve as a readily available carbon source for microbial growth. However, because seed-borne bacteria colonized the developing rhizosphere first, we believe they had early access to these plant-derived sugars, allowing them to rapidly colonize and likely deplete these key resources before soil microbes arrived and could compete. This priority effect ecological principle, gave seed-borne bacteria a decisive advantage, enabling them to outcompete late-arriving soil microbes and dominate the rhizosphere microbiome.
But it didn’t stop there. Interestingly, we also found that some seed bacteria specialized in metabolizing key plant-exuded disaccharides—sucrose, maltose, trehalose, and cellobiose. This specialized metabolic ability likely reduces direct competition by dividing the resource landscape, a phenomenon known as niche partitioning. Their specialization allows them to coexist even in the presence of strong competition for simple sugars and reinforces their establishment in the wheat rhizosphere.
Niche facilitation: Microbes helping microbes
Another fascinating aspect of this microbial formation process was niche facilitation—a phenomenon where one microbial species creates conditions that support others. Using in vitro experiments, we demonstrated that some seed-borne bacteria brake down disaccharides into simpler monosaccharides that are then used by other bacteria. These helper seed bacteria effectively supported the growth of their microbial partners, thereby strengthening the community as a whole. This cooperative dynamic provided further evidence that the assembly of the rhizosphere microbiome is not just a random process, but a structured, interactive system shaped by both competition and cooperation among different bacteria and modulated by the plant host—after all, the plant releases these carbon-rich molecules to set the whole play in motion (Garrido-Sanz et al., 2023).
Reproducibility: can we predictably shape plant microbiomes?
A key takeaway from our study is the discovery that microbiome assembly was highly reproducible across sequential plantings. Despite the inherent complexity of microbial communities, our sequential propagation approach consistently produced a similar rhizosphere microbiome forming over time. This reproducibility is critical because it suggests that, under the right conditions, we can indeed guide microbiome assembly toward stable states. Furthermore, we were able to store and recover the complex rhizosphere community, maintaining the same composition and structure. This is a crucial asset for experimental research, as it allows us to manipulate individual elements while leaving the rest of the system unchanged, providing a powerful framework for testing microbiome dynamics and interactions.
Implications for future research and agriculture
Our findings redefine the role of seed microbiota in plant-microbe interactions, demonstrating that plant-associated microbial communities are not simply passively assembled from the environment, but are actively shaped by inherited microbial lineages. These findings have important implications for microbiome engineering, as we can rationally harness the natural priority effects of seed-borne microbes to favor the assembly of plant-beneficial bacteria within the plant’s natural microbiome. In this way, we can steer rhizosphere microbiomes toward compositions that enhance plant health and reduce chemical inputs.
We set out to explore how soil and seed microbiomes coalesce in the rhizosphere, expecting soil microbes to play the dominant role. Instead, we found that seed-borne bacteria act as key architects of the root microbiome, shaping microbial succession through priority effects, niche partitioning, and facilitation. This discovery not only advances our understanding of microbiome assembly but also opens up exciting possibilities for microbiome-driven agricultural innovation. As we continue to unlock the secrets of plant-microbe interactions, we move closer to a future where we can actively shape microbial communities to support healthier, more resilient crops.
Cover photo: Wheat field in the St. Maurice region, Valais, Switzerland. Photographed by Daniel Garrido-Sanz.
References
Burz SD, Causevic S, Dal Co A, Dmitrijeva M, Engel P, Garrido-Sanz D, et al. (2023). From microbiome composition to functional engineering, one step at a time. Microbiology and Molecular Biology Reviews, 87(4):e00063-23. DOI: doi.org/10.1128/mmbr.00063-23
Garrido-Sanz D, Keel C (2025). Seed-borne bacteria drive wheat rhizosphere microbiome assembly via niche partitioning and facilitation. Nature Microbiology, doi.org/10.1038/s41564-025-01973-1
Garrido-Sanz D, Čaušević S, Vacheron J, Heiman CM, Sentchilo V, van der Meer JR, Keel C. (2023). Changes in structure and assembly of a species-rich soil natural community with contrasting nutrient availability upon establishment of a plant-beneficial Pseudomonas in the wheat rhizosphere. Microbiome, 11(1):214. DOI: doi.org/10.1186/s40168-023-01660-5
Harmsen N, Vesga P, Glauser G, Klötzli F, Heiman CM, Altenried A, Vacheron J, Muller D, Moënne-Loccoz Y, Steinger T, Keel C, Garrido-Sanz D. (2024). Natural plant disease suppressiveness in soils extends to insect pest control. Microbiome, 12(1):127. DOI: doi.org/10.1186/s40168-024-01841-w
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in