Shaking of our genetic foundation—how SARS-CoV-2 infection alters the 3D genome and what that means for our immune response
Published in Microbiology
Explore the Research

SARS-CoV-2 restructures host chromatin architecture - Nature Microbiology
Hi-C 3.0 and ChIP-seq show that SARS-CoV-2 infection alters the host cell 3D genome structure and epigenome.
The three-dimensional (3D) organization of genetic material within our cells is highly influential in regard to transcriptional activity and can be altered in response to external stimuli. Viewing infection by SARS-CoV-2 as one such stimulus, we set out to investigate how this pandemic-causing virus might impact features of host chromatin and how those impacts, in turn, contribute to defects in a successful anti-viral response.
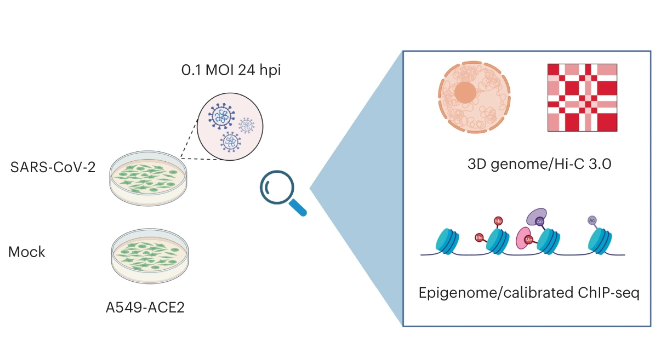
Previous work has identified several DNA viruses and retroviruses as manipulators of host transcription via chromatin remodeling and modification (KSHV, HPV, EBV, HIV, etc.). A much smaller body of evidence has also begun to implicate (non-retrovirus) RNA viruses in this context, with some of the most studied thus far being influenza, hepatitis C, and RSV. When the pandemic began over three years ago now, we began to question if this novel coronavirus might have similar or potentially more severe effects on host chromatin that could help to explain the immune dysregulation being observed clinically. Indeed, upon investigation into host chromatin structure after SARS-CoV-2 infection of A549 cells, we found significant alterations to several organizational features that could be linked to transcriptional perturbations. More specifically, we observed weakening and mixing of chromatin compartments, loss of physical interactions within topologically associated domains (TADs), and gain/loss of histone tail modifications at critical loci in the anti-viral response.
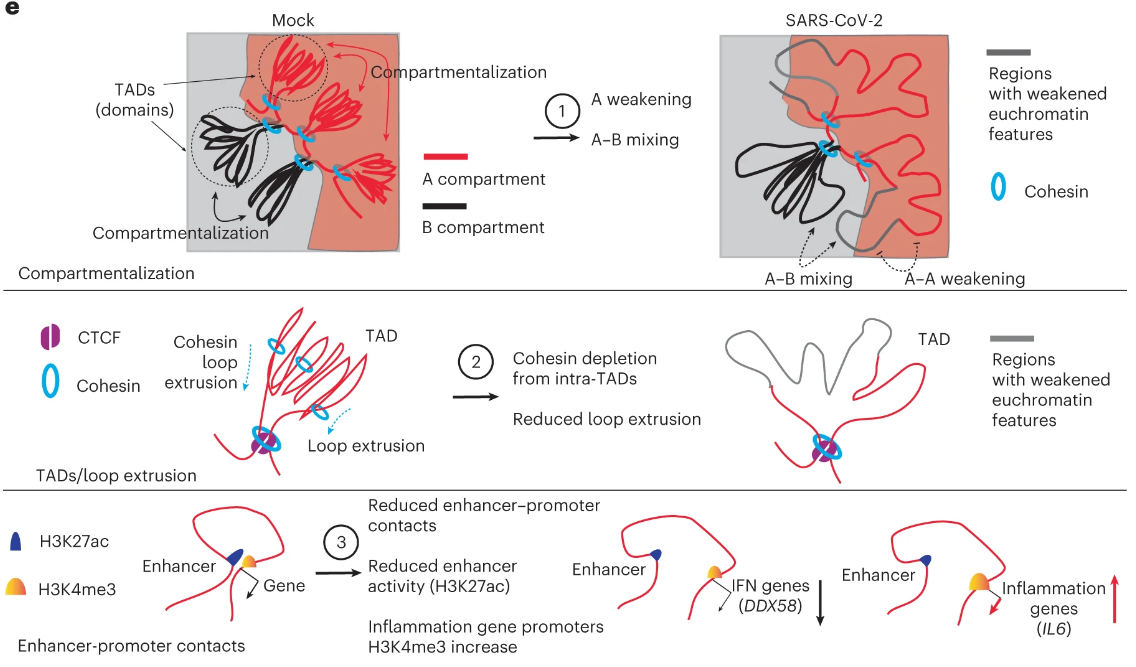
Chromatin compartmentalization refers to the typical separation of transcriptionally active and transcriptionally repressed regions (euchromatin and heterochromatin, respectively). Weakening and mixing of these compartments after SARS-CoV-2 infection indicates that mechanisms of chromatin compartmentalization have become defective and that these regions have begun to lose identity. Changes to histone modifications in these regions contribute to this effect. Another key observation was the loss of intra-TAD interactions, mediated by a loss of cohesin residency. TADs represent regions of preferential physical interaction between genetic sequences and are thought to be formed by DNA extrusion through a ring complex called cohesin. One major function of TAD formation and loop extrusion is to bring transcriptional regulatory sequences that are distant in the linear sequence into proximity in 3D. We found that after SARS-CoV-2 infection, this cohesin structure was depleted from the TADs and some genes critical to the interferon response subsequently lost their enhancer-promoter contacts. This results in reduced transcriptional activity and is indicative of a weakened immune response. Finally, we found increased deposition of an activating histone modification (H3K4me3) at the promoters of several pro-inflammatory genes that, when left in a state of dysregulation, can lead to what is known as a ‘cytokine storm’.
At each level of genome organization that we explored, we found that the observed changes were more severe in the SARS-CoV-2 infected condition than in any of our other viral infections or immune stimulants. This argues that the observed phenomenon is somewhat unique to SARS-CoV-2 and that these changes are not a result of a more general immune response. Although the exact mechanism by which the virus achieves this manipulation remains to be determined, these findings nevertheless represent an important RNA virus-mediated perturbation to host chromatin with significant clinical implications.
Since completion of this work, which focuses on what occurs immediately upon acute infection, the ever-evolving conversation surrounding ‘long-COVID’ or PASC (post-acute sequelae of COVID-19) has inspired us to consider the long-term effects of chromatin alterations—especially those that interfere with the delicate balance of the immune system. Further investigation into these ideas is certainly required, however, we believe changes in this environment could represent a possible link between what happens during acute infection and the persistent symptoms we can now see in cases of long-COVID.
References
- Paschos, K. & Allday, M. J. Epigenetic reprogramming of host genes in viral and microbial pathogenesis. Trends in Microbiology 18, 439–447 (2010).
- Marazzi, I. et al. Suppression of the antiviral response by an influenza histone mimic. Nature 483, 428–433 (2012).
- Perez, S. et al. Hepatitis C virus leaves an epigenetic signature post cure of infection by direct-acting antivirals. PLOS Genetics 15, e1008181 (2019).
- Xu, X., Qiao, D., Mann, M., Garofalo, R. P. & Brasier, A. R. Respiratory Syncytial Virus Infection Induces Chromatin Remodeling to Activate Growth Factor and Extracellular Matrix Secretion Pathways. Viruses 12, 804 (2020).
- Choutka, J., Jansari, V., Hornig, M. & Iwasaki, A. Unexplained post-acute infection syndromes. Nat Med 28, 911–923 (2022).
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in