Significance of hereditary gene alterations for the pathogenesis of adult bone marrow failure versus myeloid neoplasia
Published in Cancer
Growing recognition of the role of germline variants in adult bone marrow failure and myeloid neoplasia
Broader genetic screening has led to the growing recognition of the role of germline (GL) variants associated with adult bone marrow failure (BMF) and myeloid neoplasia (MN) not exclusively in children and young adults. Historically, inherited genetic factors are often suspected and frequently identified in children with BMF syndromes1. In contrast, most of BMF and MN diagnosed in adulthood have been assumed not to be driven by deleterious GL variants, except in cases with a strong family history of BMF or leukemia, or with a young age at diagnosis2, 3. Recently, the application of whole exome/genome sequencing has demonstrated GL traits in a variety of hematological malignancies, chiefly of myeloid origin, dismantling the classical notion that adult diseases, unlike most pediatric BMF syndromes, are always acquired. For instance, a genetic screening of smaller cohorts of adults aged 18-40 years diagnosed with aplastic anemia or myelodysplastic neoplasm revealed that 15%-19% of patients harbored deleterious GL variants4. In addition, the BEAT AML study identified that 13.6% of patients unselected by family history have pathogenic (P) or likely pathogenic (LP) variants in cancer-predisposition genes5.
Due to differences in disease latency and phenotypes, reduced penetrance, and heterogeneous application of routine genetic testing, some carriers of GL leukemia and cancer predisposing alterations for the development of MN may not be recognized, thereby hindering prevalence estimates in adult populations. Moreover, absent or incomplete family history and competing mortalities may prevent the precise estimationof the actual etiologic fraction of hereditary gene alterations in the pathogenesis of adult BMF and MN. At a genetic level, carriers of otherwise known recessive traits may not be entirely free of risk, which can be only assessed in long-term survivors and may become manifest in specific clinical situations. Based on the increased prevalence of GL alterations in adult patients, we suspect that the role of hereditary factors in the pathogenesis of adult BMF and MN is greatly underestimated.
We previously estimated the frequency of rare GL variants in BMF and MN cases. However, the gene panel used in our previous studies included only a small number of genes6, 7. Although a complete screen for all known GL alterations is not feasible, the study of a broad panel of rationally selected genes may reveal the extent to which we underestimate how ubiquitous hereditary factors are in adults and how they affect the course of the disease.
Our findings
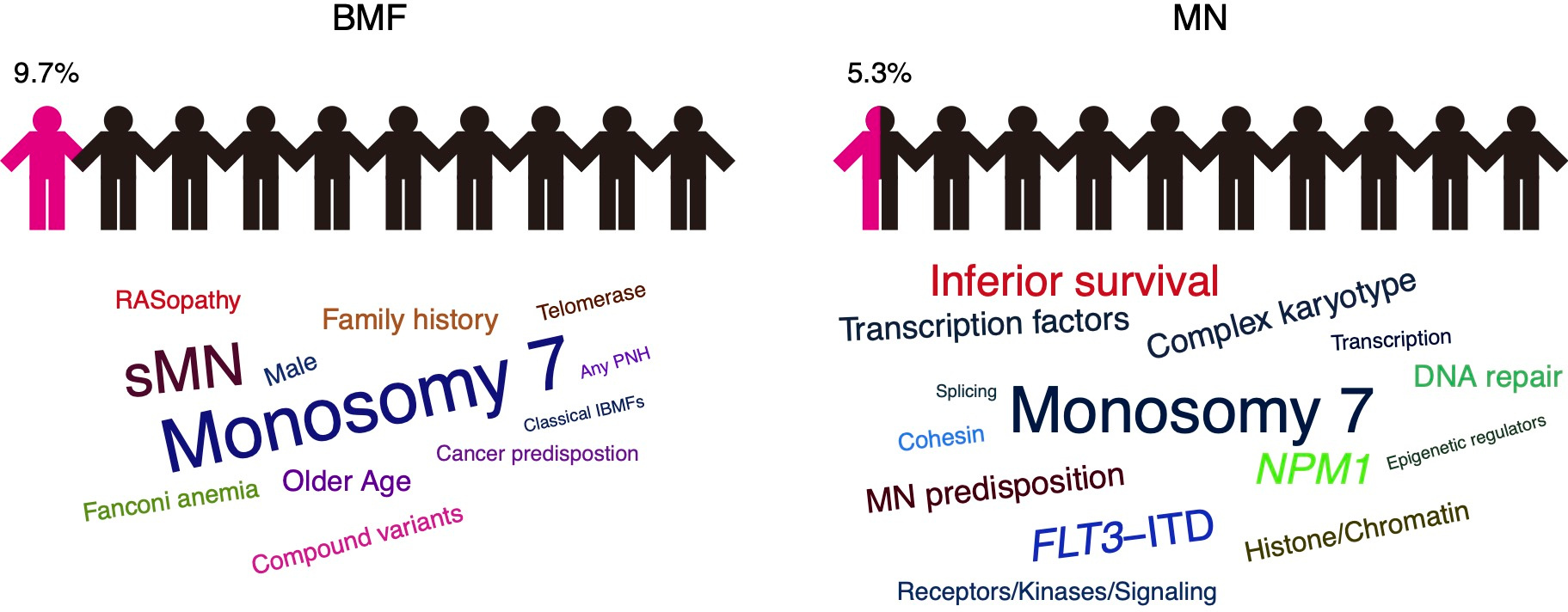
In this study, we screened 350 adult BMF and 2658 MN cases using a GL variant panel of 163 selected genes to adult to assess the importance of germline genetics and its impact on disease phenotype and prognosis.
In our cohort, up to 9.7% of BMF and 5.3% of MN cases carried GL variants. In the BMF subcohort, secondary MN (sMN) progression and monosomy 7 acquisition were associated with the presence of pathogenic and likely pathogenic (P/LP) variants. In the MN subcohort, cases harboring P/LP variants showed higher frequencies of monosomy 7 and complex karyotypes. Notably, FLT3-ITD, NPM1, and transcription factor mutations positively correlated with P/LP variants in MN cases. Similar to BMF, MN cases with P/LP variants also had a higher frequency of monosomy 7. Both cohorts also included heterozygous carriers of recessive traits, suggesting their contribution to the risk of BMF and MN.
By gene category, variants of Fanconi anemia (FA) gene family had the highest-frequency for both BMF and MN cases as they were found in 4.9% and 1.7% cases, respectively. When compared to MN, BMF patients showed higher frequencies of alterations in most gene categories with particularly higher frequencies of P/LP variants in the RAS family and telomerase machinery genes. Conversely, MN tended to have higher frequencies of P/LP alterations in receptors/kinases/signaling, cohesins, and transcription factor genes. In addition, about 1.4% of BMF and 0.19% of MN cases harbored multiple germline variants affecting often functionally related genes as compound heterozygous.
Then we also evaluated the effects of the identified GL predisposition traits on patients’ clinical outcomes. BMF cases with P/LP variants had similar overall and progression-free survival when compared to non-carriers. In contrast, P/LP variant carriers in the MN cases showed a shorter overall survival as opposed to non-carriers. When studying the potential effect of inheritance patterns (autosomal dominant/recessive), MN patients carrying P/LP variants inherited in an autosomal dominant fashion showed shorter overall survival compared to non-carrier cases.
For the better future
Our results revealed that GL variants contributed to a greater extent to the pathogenesis and development of BMF than to that of MN. Classically, heterozygous carriers for each FA gene mutation except for FANCB and FANCR have long been considered to be asymptomatic carriers, because FA genes are inherited in a recessive manner. Nonetheless, our cohort included a significant number of heterozygous carriers of recessive traits, so that an association with the development of MN and BMF in heterozygous carriers cannot be ruled out. Advances in medical technology have overcome various diseases and greatly extended our life span. However, in the future long-term life with reduced competing mortality may phenotypically unveil familial syndromes based on GL predisposition.
While MN cases with germline variants experienced a significantly shorter overall survival as compared to non-carrier cases. the prognosis of BMF cases was not affected by the presence of P/LP variants. However, the presence of P/LP variants was associated with sMN progression and monosomy 7 acquisition, which would clearly have a negative effect on survival. Because the disease anticipation from the time of BMF diagnosis is highly variable likely due to additional genetic diversity and competing morbidities, the impact of P/LP may be obscured in this context.
Finally, existing risk classification systems of adult myeloid malignancies are based on cytogenetic and molecular features in addition to clinical risk factors. As it has been shown, GL DDX41-related myeloid malignancies represent a specific nosologic subentity. Consequently, GL predisposition should be incorporated into the risk classification. The appreciation of GL predispositions for BMF and MN can significantly change behavior in clinical settings. More and more genetic screening will be done in the future. This includes surveillance for specific organ dysfunction (e.g., FA genes), genetic counseling, and, of outmost importance, the choice of donors in patients eligible for hematopoietic stem cell transplantation, i.e., it may be not appropriate to use relatives as donors if inherited traits segregate within the family.
References
- Keel SB, Scott A, Sanchez-Bonilla M, Ho PA, Gulsuner S, Pritchard CC, et al. Genetic features of myelodysplastic syndrome and aplastic anemia in pediatric and young adult patients. Haematologica 2016 11; 101(11): 1343-1350.
- Feurstein S, Drazer M, Godley LA. Germline predisposition to hematopoietic malignancies. Hum Mol Genet 2021 10 01; 30(20): R225-R235.
- Rio-Machin A, Vulliamy T, Hug N, Walne A, Tawana K, Cardoso S, et al. The complex genetic landscape of familial MDS and AML reveals pathogenic germline variants. Nat Commun 2020 02 25; 11(1): 1044.
- Feurstein S, Churpek JE, Walsh T, Keel S, Hakkarainen M, Schroeder T, et al. Germline variants drive myelodysplastic syndrome in young adults. Leukemia 2021 08; 35(8): 2439-2444.
- Yang F, Long N, Anekpuritanang T, Bottomly D, Savage JC, Lee T, et al. Identification and prioritization of myeloid malignancy germline variants in a large cohort of adult AML patients. Blood 2021 Sep 05.
- Przychodzen B, Makishima H, Sekeres MA, Balasubramanian SK, Thota S, Patel BJ, et al. Fanconi Anemia germline variants as susceptibility factors in aplastic anemia, MDS and AML. Oncotarget 2018 Jan 05; 9(2): 2050-2057.
- Li ST, Wang J, Wei R, Shi R, Adema V, Nagata Y, et al. Rare germline variant contributions to myeloid malignancy susceptibility. Leukemia 2020 06; 34(6): 1675-1678.
Follow the Topic
-
Leukemia

This journal publishes high quality, peer reviewed research that covers all aspects of the research and treatment of leukemia and allied diseases. Topics of interest include oncogenes, growth factors, stem cells, leukemia genomics, cell cycle, signal transduction and molecular targets for therapy.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in