Silencing the Saboteurs - The IL1R1+ CAFs Fueling Colorectal Cancer Growth
Published in Cancer

Introduction
Colorectal cancer (CRC) is a major healthcare challenge worldwide, with nearly 2 million people being diagnosed each year1. While the effectiveness of immune checkpoint inhibitors (ICI) has revolutionized the treatment landscape in specific solid tumors such as melanoma and lung cancer, they show little clinical efficacy in most subsets of CRC, leaving patients with few effective treatment options. This has led CRC tumors to be often described as immunologically “cold”, meaning that immune cell infiltration is either low, or limited to immunosuppressive subsets which are unable to sustain effective immune responses. This outlines a clear therapeutic need, further reinforced by the molecular classification of CRC, which has identified a colorectal cancer subtype (CMS4) defined by a high level of stromal infiltration and associated with worse therapy response and prognosis2.
Cancer associated fibroblasts (CAFs) are the most abundant cell type in the tumor stroma. They have been associated with the majority of cancer hallmarks, contributing among other things to tumor proliferation, angiogenesis, therapy resistance and modulation of tumor immunity3. They are highly plastic, being able to react to environmental queues from other actors within the tumor microenvironment (TME). CAFs are often classified into 3 main categories: (1) myofibroblastic myCAFs responsible for contractile activity and extracellular matrix remodeling, (2) inflammatory iCAFs able to secrete large quantities of cytokines, and (3) antigen presenting apCAFs characterized by antigen presentation machinery expression. Due to their high plasticity and functional heterogeneity, further subclassification of CAFs is needed. Here, we describe a novel iCAF subset characterized by elevated expression of the IL1R1 surface receptor that can enhance tumor growth and maintain an immunosuppressive TME.
"Investigating the Dynamic Landscape of CAFs in CRC Tumors"
The starting point of our investigation was the observation that IL1R1 expression is increased on CAFs when compared to normal fibroblasts situated outside of the tumor margin. Further investigation in several scRNA-Seq CRC datasets led to the discovery that IL1R1 expression and elevated IL-1β signaling characterizes a subset of iCAFs, the CAF subtype known for its immunomodulatory properties and cytokine secretion. IL1R1 expression in CAFs positively correlates with expression of the iCAF markers podoplanin and fibroblast activation protein (FAP). IL1R1+ CAFs are further distinguished by an increased responsiveness to IL-1 compared to normal fibroblasts as well as their ability to secrete inflammatory cytokines such as IL-6 and IL-8, extracellular matrix remodeling molecules such as MMP2 and protumorigenic molecules such as CXCL1 and IGF1.
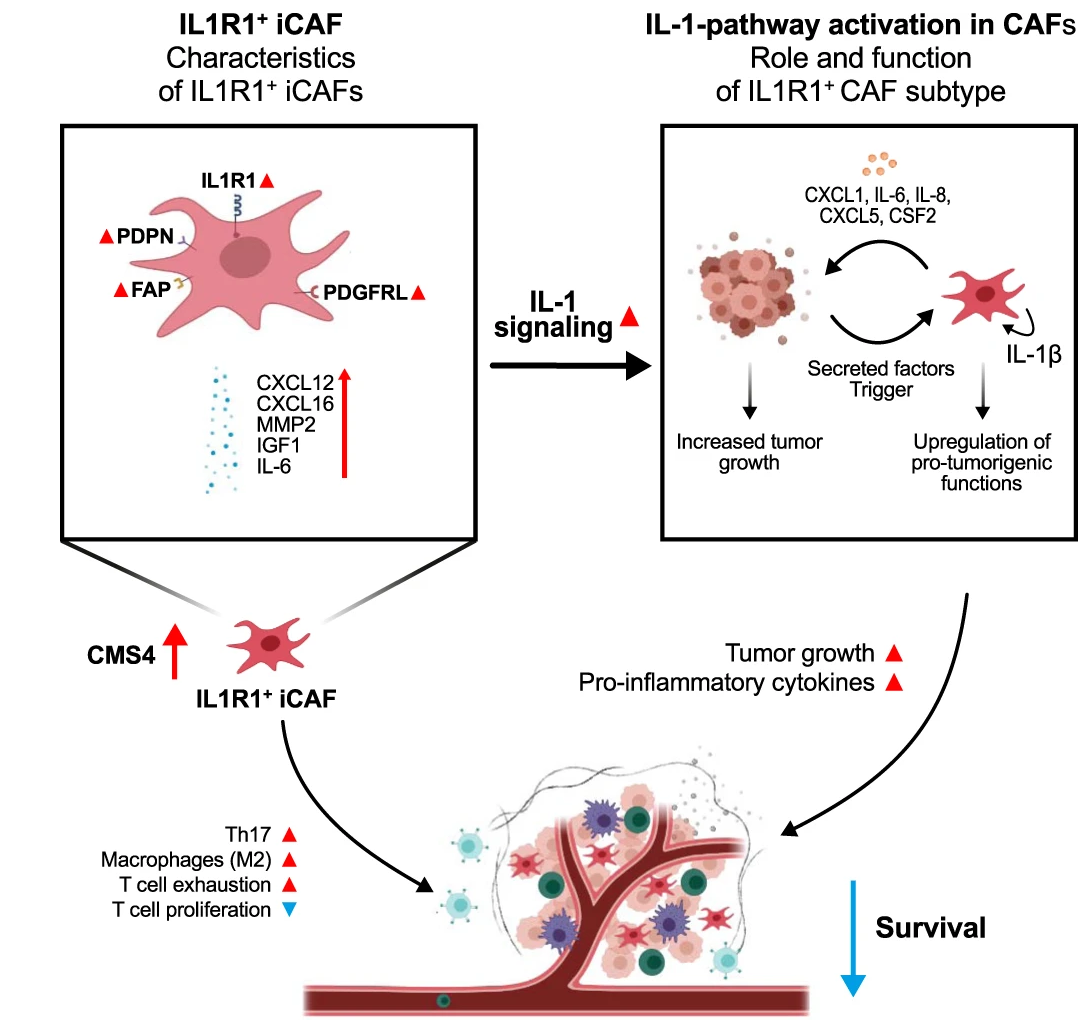
"Decoding the IL1R1-IL-1β axis in iCAFs: Insights into Tumorigenic Signaling"
Intrigued by the tumor promoting secretome of IL-1β activated CAFs, we validated that they could indeed act in a pro-tumorigenic fashion using both in-vivo and in-vitro approaches.
In-vitro, we observed that soluble factors secreted by IL-1β activated CAFs directly increased tumor spheroid growth, and further that direct coculture of IL-1β prompted CAFs with tumor spheroids in collagen matrices led to an increase in outgrowth of the invading tumor spheroids. Similarly, IL-1 pathway blockade reduced tumor outgrowth in such three-dimensional organotypic assays.
In-vivo, subcutaneous injection of colorectal cancer cells into genetically engineered mice bearing a conditional knockout of IL1R1 in stromal cells revealed a notable impairment in tumor progression, while pharmacological blockade of IL1 signaling with the decoy receptor Anakinra showed similar results.
“Unraveling the Immunosuppressive Effects of IL1R1+ CAFs in CRC”
Over recent years, CAFs have been emerging as important modulators of the TME immune compartment4. The marked expression of several proinflammatory cytokines and chemokines by IL1R1+ CAF led us to further investigate the immunomodulatory effects of this iCAF subset.
In a naïve approach, we initially screened the well-established Cancer Genome Atlas (TCGA) CRC dataset. In this bulk RNA-Seq dataset, we observed that tumors from CMS4 patients with high levels of IL1R1 expression showed increased expression of markers associated with T-cell exhaustion, immune checkpoint inhibition and pro-tumorigenic macrophage polarization. In the Marisa dataset, we found that high IL1R1 expression in CMS4 patients correlated with significantly worsened prognosis.
We were able to recapitulate these findings in-vitro, where we could show that antigen specific T-cell proliferation was reduced when T-cells were cocultured with IL-1β pretreated fibroblasts. Similarly, coculture of macrophages with IL-1β prompted CAFs resulted in a drastic increase of M2 polarization associated markers. This immunosuppressive effect of IL1R1 CAFs was further reinforced by increased expression of the immune checkpoint inhibitor PD-L1 on CAFs stimulated with IL-1β.
Similarly, we could show that fibroblast specific IL1R1-KO reduced PD-L1 expression in murine tumors in-vivo, pointing towards a less immunosuppressive TME. Fibroblast specific IL1R1 KO as well as Anakinra treatment also reduced IL-17 secreting Th17 T-cell infiltration in mouse tumors, a population mostly known for its pro-tumorigenic effects in CRC.5
Personal reflection - A story of firsts
Our work with CAFs began as it so often does in science, by coincidence. As we were establishing biospecimen handling and isolation protocols for our incipient biobank, we noticed that in our attempts to isolate tumor initiating cancer stem cells, we would often end up isolating populations with fibroblastic morphology from our patient samples. This led us to investigate the role that this population might have in shaping the tumor microenvironment (TME).
The realization of this paper marked a series of “firsts” for our relatively young group at the time. Our introduction to the realm of CAFs included mastering their isolation and cultivation, offering us a gateway into new cellular interactions.
Collaborating with Pr. Helmut Dolznig in Vienna was a pivotal juncture, as his expertise in diverse CAF functional assays and willingness to host collaborative visits greatly expanded our understanding of their role. Broadening our experimental scope, we also tackled conditional knock-out mouse models for the first time.
Conclusion and outlook
In summary, we found that IL1R1+ CAFs drive immunosuppression and tumor growth both in-vitro and in-vivo, contributing to CRC tumor progression.
Following recent studies highlighting the role played by IL-1α signaling CAFs in therapy resistant rectal cancer6, sparking a clinical trial combining IL1R1 antagonist Anakinra with radiotherapy7 , our findings further support the impact that IL‑1 signaling in the stromal compartment has in shaping the TME . The almost exclusive expression of IL1R1 on fibroblasts in the gut makes it a promising candidate for targeted therapeutic interventions in fibrotic CRC tumors. The fact that IL‑1 signaling in CAF also contribute to immune checkpoint expression in tumors also opens the door to combination therapies with immune checkpoint inhibitors, potentially providing new therapeutic options to CMS4 patients who typically fail to respond to immunotherapy.
- Xi, Y. & Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 14, 101174 (2021).
- Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356 (2015).
- Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020).
- Crosstalk between cancer-associated fibroblasts and immune cells in cancer - PubMed. https://pubmed.ncbi.nlm.nih.gov/31642585/.
- Razi, S., Baradaran Noveiry, B., Keshavarz-Fathi, M. & Rezaei, N. IL-17 and colorectal cancer: From carcinogenesis to treatment. Cytokine 116, 7–12 (2019).
- Nicolas, A. M. et al. Inflammatory fibroblasts mediate resistance to neoadjuvant therapy in rectal cancer. Cancer Cell 40, 168-184.e13 (2022).
- Fleischmann, M. et al. ACO/ARO/AIO-21 - Capecitabine-based chemoradiotherapy in combination with the IL-1 receptor antagonist anakinra for rectal cancer Patients: A phase I trial of the German rectal cancer study group. Clin. Transl. Radiat. Oncol. 34, 99–106 (2022).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in