Small molecule, BIG impact: how glutathione metabolism affects fungal virulence via redox regulation
Published in Microbiology

Being a pathogen is no easy task – it requires the exquisite coordination of attack and defense systems that protect the pathogen from the harsh host environment and help facilitate infection. The fungal pathogen Cryptococcus neoformans, however, is particularly adept at navigating such hostile environments. Over millennia, C. neoformans has developed an arsenal of mechanisms that help it survive, proliferate and cause disease in mammalian hosts. These mechanisms include a thick polysaccharide capsule that surrounds the cell and melanin, a dark pigment that coats the exterior of the cell and helps neutralize offending oxidants. Melanin is particularly important for defending cryptococcal cells during infection, as it can scavenge harmful free radicals and protect cells from oxidative bursts during engulfment by host innate immune cells. Melanin synthesis is also linked to cryptococcal meningoencephalitis – an often-fatal stage of infection in which the fungus infiltrates the central nervous system (CNS). C. neoformans can then convert catecholamines (dopamine, norepinephrine, and epinephrine) found in the CNS into melanin, further fortifying it from host defenses and advancing disease severity.
However, because melanin synthesis depends on polymerization of free radicals it has been difficult to study; the compound also requires complex regulatory networks to prevent cell toxicity. Therefore, exploring the mechanisms behind the redox-mediated processes involved in melanin production are crucial for understanding virulence and defense strategies of pathogenic fungi – organisms that are becoming increasingly prevalent alongside a continually growing at-risk population. Though the global incidence of cryptococcosis alone has declined with increased availability of antifungals and antiretroviral therapies (ART), the global burden of disease is still substantial, especially among countries with a high prevalence of HIV/AIDS. An estimated 152,000 cases of cryptococcal meningitis occur annually, resulting in 112,000 deaths – 71,000 of which occur in sub-Saharan Africa, the region with the highest incidence of cryptococcosis worldwide. Globally, cryptococcal disease accounts for 19% of AIDS-related deaths. The recent designation of C. neoformans as a fungal pathogen of critical importance by the World Health Organization also highlights the urgent need to understand cryptococcal infection – a disease with limited treatment options and no vaccine. Developing treatments for infectious diseases such as cryptococcosis (many of which commonly occur in developing nations) is critical for lowering the incidence and severity of these diseases.
Our work in the Kronstad lab employs omic and genetic approaches that help unravel the mysteries behind the factors responsible for cryptococcal virulence, including how redox-mediated processes influence melanin formation. Recently, we demonstrated that metabolism of glutathione (GSH), a potent antioxidant that occurs abundantly in most cell types of many organisms, is inextricably linked to the processes that facilitate melanin formation. One of our key findings was that genetic mutants of C. neoformans lacking a GSH biosynthesis gene had severely reduced growth and attenuated virulence in mice. This mutation precluded synthesis of GSH by C. neoformans and had unexpected impacts on the extracellular environment. Namely, the extracellular milieu was highly acidified in GSH mutants, and these extracellular changes blocked melanin production (which occurs at the cell wall). Together, we surmised that a precise redox balance (or a “Goldilocks” zone) was required for melanin synthesis to occur, and changes to this balance could block or impair melanogenesis. Changing redox conditions not only impacted melanin formation, but also the response to external stress. Though mutants lacked GSH (a strong antioxidant), they were less susceptible to modest oxidative stress by hydrogen peroxide, and we attributed these changes to extracellular acidification and a low extracellular pH.
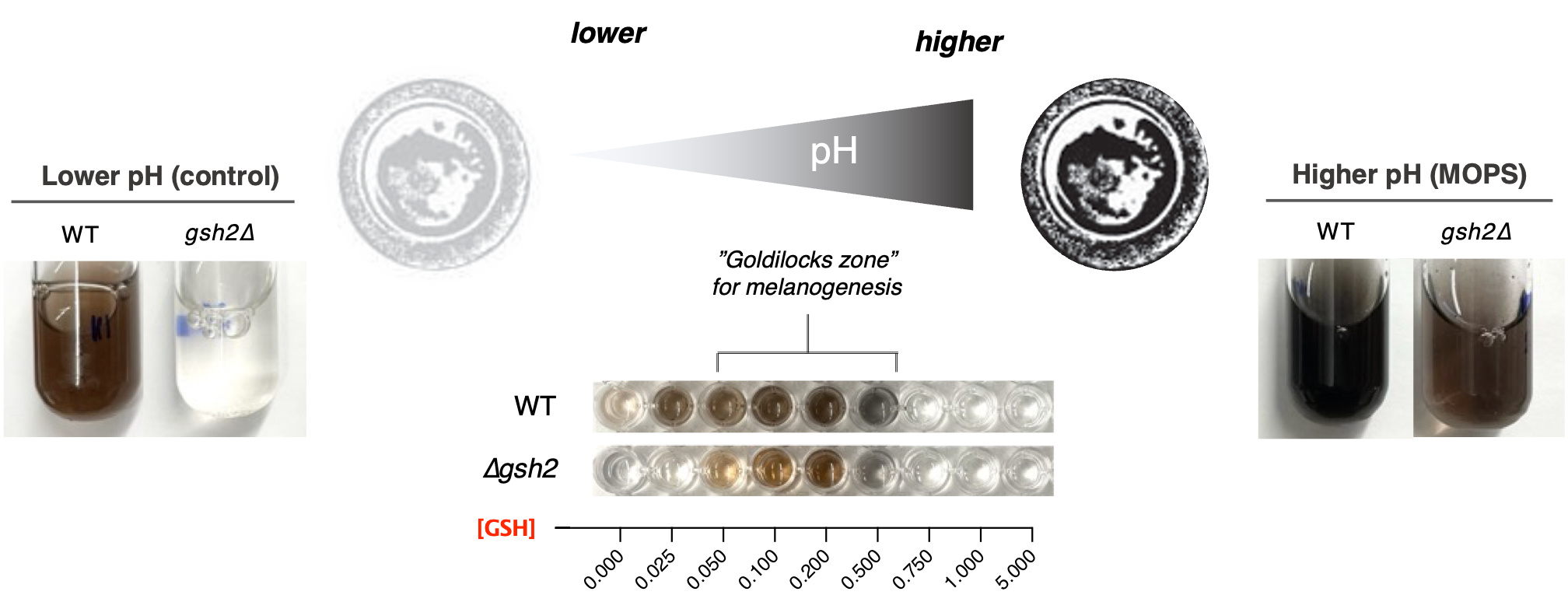
In addition to the melanin and redox changes observed, C. neoformans GSH mutants had reduced migration to the brains of mice. In part, this can be explained by lower phagocytic uptake of mutants, which can impede traversal of the blood-brain barrier via a “Trojan horse” mechanism – a tactic by which C. neoformans invades the CNS by concealing itself within host immune cells. Another contributing factor is the inability of GSH mutants to form titan cells. Titan cells are large (> 10µM) cells linked to cryptococcal persistence and virulence, and their progeny can have enhanced adaptation to stressors in the host environment. Titan cells also skew host immunity to favour cryptococcal survival and dissemination, thereby enhancing disease. This lack of titan cell formation and the reduced cryptococcal cell migration observed with GSH mutants suggest a complex interplay between redox regulation, host immunity and progression of disease.
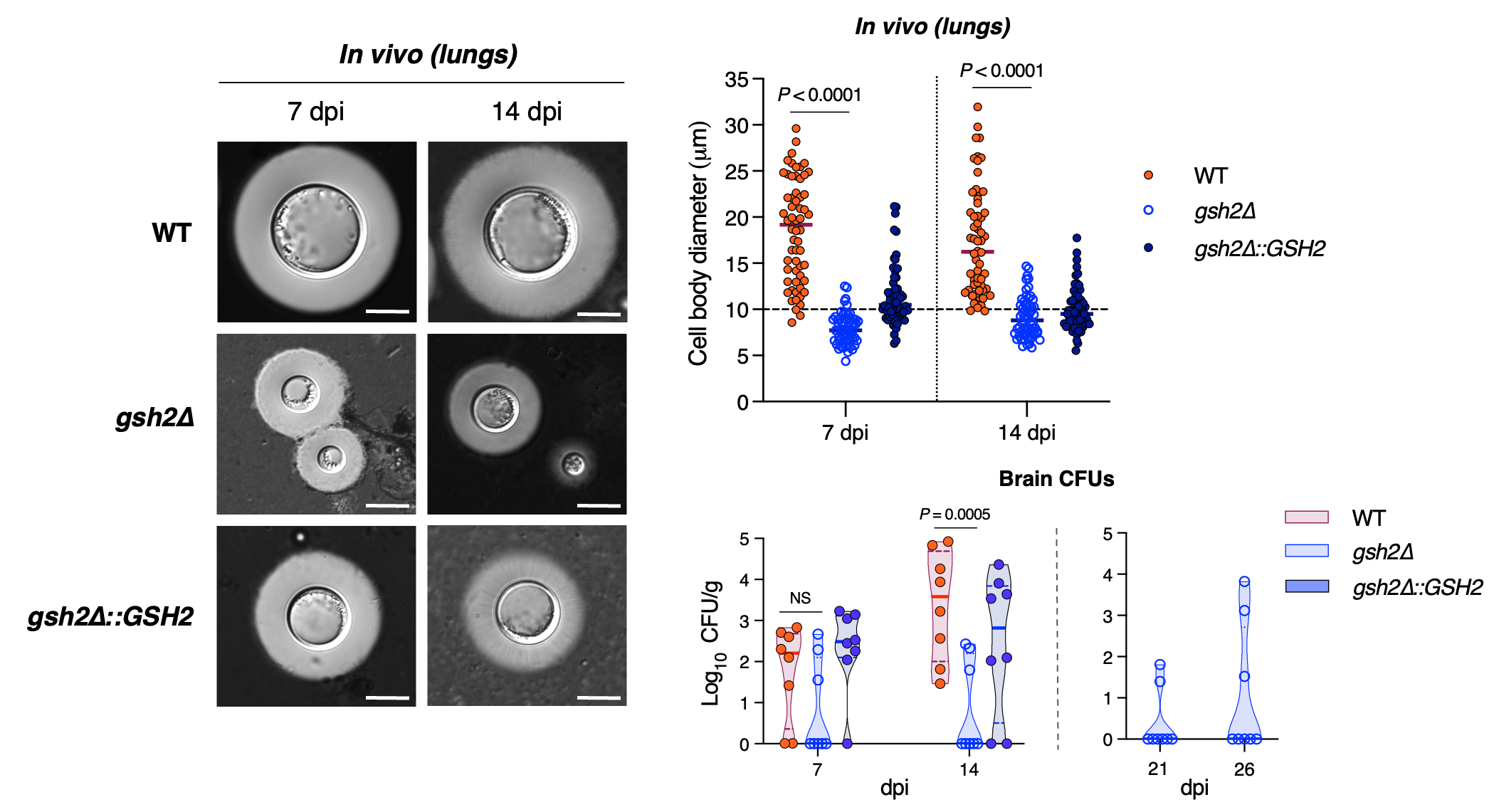
Collectively, our findings demonstrate a key role for regulation of redox homeostasis in pathogen survival and disease. In C. neoformans, GSH has a particular role in establishing the ‘Goldilocks’ conditions needed for melanin production – a phenomenon that until this point had not been shown in a human fungal pathogen. Disturbing this redox balance influenced major virulence traits, including melanin and titan cell formation, dissemination to the brain and the extracellular composition of cells. This research provides a framework for understanding how microorganisms not only mitigate a stressful host environment, but also how they can manipulate the extracellular environment to support virulence. Though GSH metabolism has been targeted for human diseases including cancer, it has not been thoroughly researched for antifungal drug discovery. Thus, our findings shed light on potential new avenues for combating fungal pathogenesis.
References:
Follow the Topic
-
Nature Microbiology

An online-only monthly journal interested in all aspects of microorganisms, be it their evolution, physiology and cell biology; their interactions with each other, with a host or with an environment; or their societal significance.
Related Collections
With Collections, you can get published faster and increase your visibility.
The Clinical Microbiome
Publishing Model: Hybrid
Deadline: Mar 11, 2026



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in