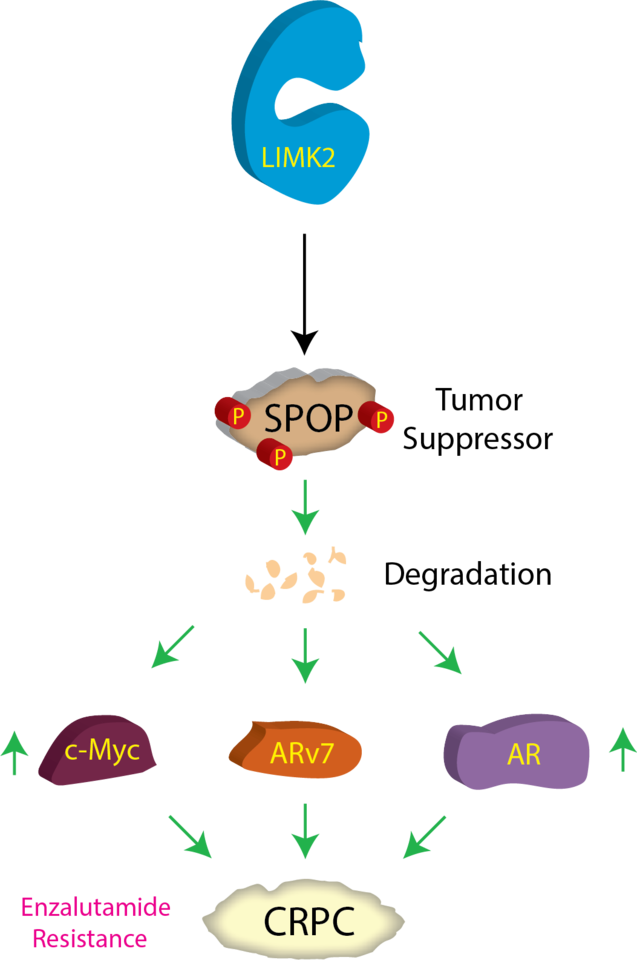
Prostate cancer (PCa) ranks 2nd in terms of cancer-related fatality in men in the US with over 191,930 estimated new cases and 33,330 projected deaths in 2020 [1]. Early-stage prostate tumors can be effectively treated by androgen-deprivation therapy (ADT) [2], nevertheless, almost all tumors eventually progress to castration-resistant PCa (CRPC), which is lethal [3, 4]. In this study, we uncovered a new mechanism by which an oncogenic kinase orchestrates castration resistance.A recent study from our laboratory had identified LIM kinase 2 (LIMK2) as a prospective clinical target for the treatment and prevention of castration-resistant prostate cancer (CRPC) [5]. In our quest for potential substrates of LIMK2 to unravel the molecular mechanisms by which it promotes CRPC pathogenesis, we have identified TWIST1 [5] and PTEN [6] as two key players. Identification of other LIMK2 substrates is expected to provide additional therapeutic targets for disease intervention. The present study centers on one such LIMK2 target, Speckle-type POZ protein (SPOP), which we identified utilizing our innovative chemical genetic technique [7]. SPOP, an E3 ubiquitin ligase adaptor, plays a pivotal role in protein ubiquitination and regulates many cellular events including regulation of cell cycle, proliferation and cell death [8]. SPOP can act as a tumor suppressor or tumor promoter depending upon the kind of cancer. In PCa, it acts as a tumor suppressor, as it degrades several tumor-promoting proteins. Thus, not surprisingly, in a vast majority of prostate tumors, SPOP’s anti-tumorigenic function is suppressed by either genomic mutations or by reduction in its protein levels. These findings underscore that SPOP downregulation is an essential step in PCa progression, although only one mechanism has been reported to date [9]. In this study, we discovered that SPOP is degraded by LIMK2, which in turn stabilizes three proteins, androgen receptor (AR), ARv7 and c-Myc, promoting CRPC.
We have delved into how phosphorylation of SPOP by LIMK2 negatively regulates its levels and stability during CRPC. Employing a range of in vitro and cellular assays, we have ascertained that LIMK2 not only directly modifies SPOP at three distinct sites leading to the loss in its stability, but SPOP also reciprocally regulates LIMK2 levels and stability, thereby suppressing LIMK2-induced cancer-related phenotypes. This molecular interplay between a tumor-suppressor protein and an oncogenic kinase has been found to be responsible for enhanced tumorigenesis, migratory ability and chemoresistance of the lethal form of prostate cancer. We also established the contribution of LIMK2-SPOP crosstalk in facilitating aggressive cancer phenotypes using a mouse model. We show that LIMK2-mediated SPOP degradation is a key mechanism that increases AR and ARv7 stability in CRPC. Since AR and ARv7 are key drivers in CRPC progression, this study uncovered a critical mechanism by which LIMK2 promotes CRPC. Thus, we believe that targeting LIMK2 provides a potent approach to retain functionally active SPOP in cells, which should not only inhibit PCa progression in patients, but may also sensitize CRPC to commonly used AR signaling inhibitors such as enzalutamide.
References:
- Siegel, R.L., Miller, K.D., Jemal, A. Cancer statistics, 2020. CA Cancer J Clin. 70, 7-30 (2020).
- Coutinho, I., Day, T.K., Tilley, W.D., Selth, L.A. Androgen receptor signaling in castration-resistant prostate cancer: a lesson in persistence. Endocrine-related cancer. 23, T179-97 (2016).
- Watson, P.A., Arora, V.K., Sawyers, C.L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer. 15, 701-11 (2015).
- Saad, F., Shore, N., Zhang, T., Sharma, S., Cho, H.K., Jacobs, I.A. Emerging therapeutic targets for patients with advanced prostate cancer. Cancer Treat Rev 76, 1-9 (2019).
- Nikhil, K., Chang. L., Viccaro, K., Jacobsen, M., McGuire, C., Satapathy, S.R., et al. Identification of LIMK2 as a therapeutic target in castration resistant prostate cancer. Cancer Lett. 448, 182-96 (2019).
- Nikhil, K., Kamra, M., Raza, A., Shah, K. Negative cross talk between LIMK2 and PTEN promotes castration resistant prostate cancer pathogenesis in cells and in vivo. Cancer Lett. Sep 12:S0304-3835(20)30472-9. Online ahead of print (2020).
- Johnson, E.O., Chang, K.H., Pablo,Y. de., Ghosh, S., Mehta, R., Badve, S. et al. PHLDA1 is a crucial negative regulator and effector of Aurora A kinase in breast cancer. J Cell Sci. 124, 2711‐22 (2011).
- Clark, A., Burleson, M. SPOP and cancer: a systematic review. Am J Cancer Res. 10,704-726 (2020).
- Nikhil, K., Kamra, M., Raza, A., Haymour, H.S., Shah, K. (2020) Molecular Interplay between AURKA and SPOP Dictates CRPC Pathogenesis via Androgen Receptor. Cancers (Basel) 212(11), E3247 (2020).
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.


Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in