Structure-function analysis of a new type of endoperoxidase
Published in Chemistry
Endoperoxide-containing natural products exhibit a wide range of biological activities and have attracted keen attention as pharmaceutical resources. Some of these compounds have been proposed to form non-enzymatically. For example, in the production of the antimalarial drug artemisinin in plants, non-enzymatic reactions with singlet oxygen (1O2), generated through light absorption by photosensitizers, have been proposed[1]. The detailed mechanisms for the enzymatically catalyzed endoperoxide formation reaction have been analyzed in only two types of enzymes, heme-dependent prostaglandin H synthase (cyclooxygenase, COX)[2] and FtmOx1, an α-ketoglutarate (α-KG)-dependent dioxygenase from Aspergillus fumigatus[3].
Our group is focused on the biosynthesis of fungal meroterpenoids, and we have identified another endoperoxidase, NvfI from Aspergillus novofumigatus[4]. NvfI catalyzes the formation of fumigatinoid A from asnovorin A by introducing “three” oxygen atoms, including an endoperoxide and a hydroxyl group at the C3' position in the biosynthesis of the complex skeletal meroterpenoid novofumigatonin, while COX and FtmOx1 install four and two oxygen atoms into their substrates, respectively (Figure 1). Although the characterization of NvfI clearly indicated that the enzyme catalyzes the incorporation of three oxygen atoms to form an endoperoxide, the detailed reaction mechanism remains unclear. Especially, the determination of whether the three oxygen atoms are introduced in a single enzyme turnover, and the identification of the catalytic residues required for the enzyme reaction still remain to be addressed.
First, to determine whether the three oxygen atoms are installed into the substrate in one round of the enzyme reaction, the stoichiometric analysis of the co-substrates α-KG and oxygen was performed. The stoichiometric analysis clearly suggested that NvfI installs “three” oxygen atoms into its substrate in a single enzyme turnover. Subsequent enzyme reactions with 18O-labeled oxygen and water molecules indicated that two O2-derived oxygen atoms and one H2O-derived oxygen atom are incorporated into the endoperoxide moiety and the C3' hydroxyl group, respectively, suggesting the existence of a long-lived ferric state during the enzyme reaction (Figure 1).
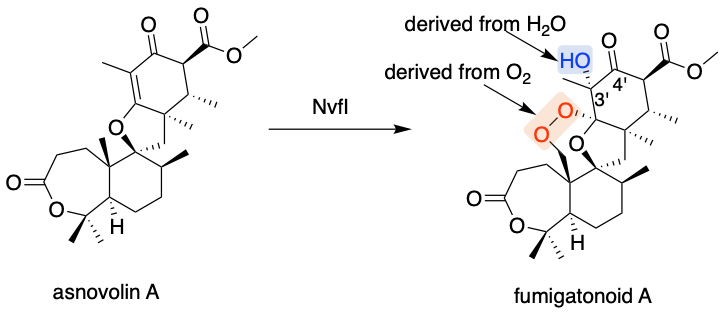
Figure 1: The reaction of NvfI.
Next, to determine how the enzyme catalyzes the incorporation of “three” oxygen atoms, we performed an X-ray crystallographic analysis of NvfI. By soaking the substrate under anaerobic conditions or using an inactive co-substrate, three different active site conformations were obtained (Figure 2). The detailed comparison of the apo and complex structures with the substrate of NvfI suggested that the dynamic conformational changes of the active site enable the repositioning of the radical intermediate, thus evading the biosynthetically undesired hydroxylation and resulting in the reaction with molecular oxygen.
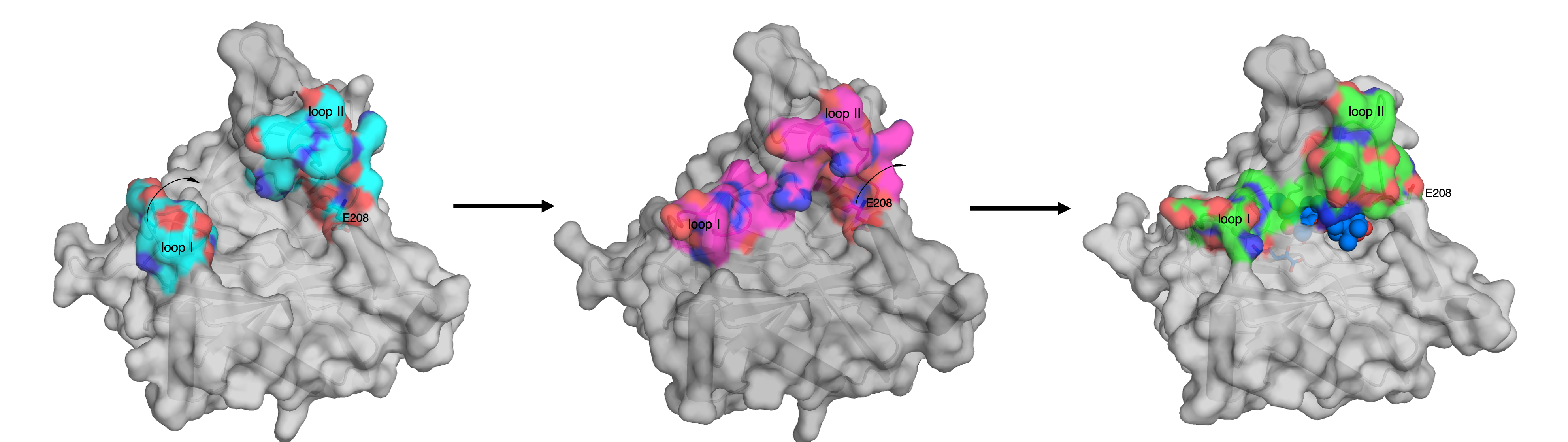
Figure 2: Conformational changes of the active site loops of NvfI.
In the enzyme reaction mechanisms of COX and FtmOx1, tyrosine residues in the active site play critical roles in the endoperoxide formation reaction through the generation of tyrosyl radicals. In contrast, the site-directed mutagenesis study of the active site residues of NvfI suggested that NvfI does not utilize any tyrosine residues to mediate the abstraction and donation of hydrogen atoms in the catalytic mechanism, indicating that the mechanism of endoperoxide formation by NvfI is significantly different from those of COX and FtmOx1.
In nature, there are still many unexplored enzymes with functions that have not yet been clarified. Therefore, the identification and mechanistic characterization of such enzymes are important for understanding how natural products are diversified. Further engineering of the substrate specificity and the enzyme reactivity of these enzymes is expected to generate biocatalysts that can be used for the oxidative modification of a wide range of natural products.
More details of this work can be found here: “Molecular insights into the endoperoxide formation by Fe(II)/α-KG-dependent oxygenase NvfI” in Nature Communications, https://www.nature.com/articles/ s41467-021-24685-6.
References:
[1] Tamez-Fernández, J. F., Melchor-Martínez, E. M., Ibarra-Rivera, T. R. & Rivas-Galindo, V. M. Plant-derived endoperoxides: structure, occurrence, and bioactivity. Phytochem. Rev. 19, 827–864 (2020).
[2] Van der Donk, W. A., Tsai, A. L. & Kulmacz, R. J. The cyclooxygenase reaction mechanism. Biochemistry 41, 15451–15458 (2002).
[3] Dunham, N. P. et al. Hydrogen donation but not abstraction by a tyrosine (Y68) during endoperoxide installation by verruculogen synthase (FtmOx1). J. Am. Chem. Soc. 141, 9964–9979 (2019).
[4] Matsuda, Y. et al. Novofumigatonin biosynthesis involves a non-heme iron-dependent endoperoxide isomerase for orthoester formation. Nat. Commun. 9, 2587 (2018).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026

Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in