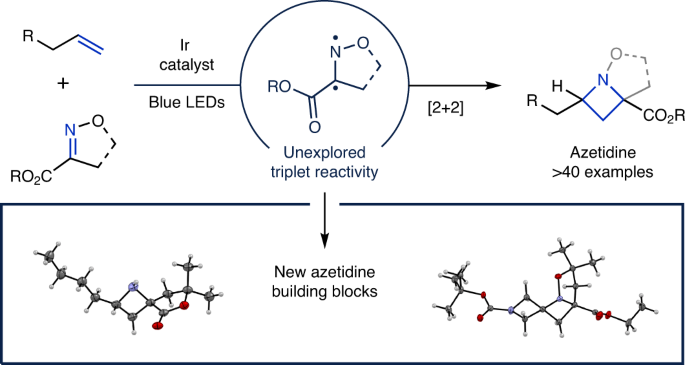
Synthesis of azetidines via visible-light-mediated intermolecular [2+2] photocycloadditions
Published in Chemistry
![Synthesis of azetidines via visible-light-mediated intermolecular [2+2] photocycloadditions](https://images.zapnito.com/cdn-cgi/image/metadata=copyright,fit=scale-down,format=auto,quality=95/https://images.zapnito.com/users/436044/posters/1598540057-74-1761/d713fe7c-b619-479d-bb22-c93a3ea691ec_large.png)
The Schindler group has recently become interested in the development of visible-light-mediated [2+2] cycloaddition reactions for the synthesis of strained heterocycles, particularly azetidines, as these scaffolds are becoming increasingly desirable for drug discovery applications. Our first foray into azetidine synthesis was the development of an intramolecular visible-light-mediated [2+2] cycloaddition method in 2019.[ref] While we were excited that this method represented the first example of a visible light mediated aza Paternò-Büchi reaction and allowed access to many azetidine products, we recognized that several challenges still needed to be overcome: 1) the current protocol is limited to intramolecular cycloadditions, as attempts to translate this method into an intermolecular variant proved unsuccessful; 2) the reaction cannot be directly carried out with commercially available reagents, and can require lengthy substrate synthesis; 3) more valuable monocyclic azetidines are difficult to access with this methodology.
With this in mind, we considered a new approach in which we would activate the oxime component instead of a styrene moiety. A literature search revealed that at least some oximes have triplet energies suitable for excitement by visible light photosensitizers, providing initial excitement for our reaction design. However, we were challenged to identify a suitable oxime substrate, as the triplet energies of only a few oximes are reported in the literature, leaving us unsure which would be appropriate for photosensitization. To answer this question, we measured the luminescence quenching of our photocatalyst for a variety of different substrates. This study allowed us to narrow down the number of potential substrates, by determining which were able to interact with the photocatalyst. Interestingly, we discovered that even though many substrates quenched the photocatalyst, most of them did not engage in the cycloaddition. However, we were ultimately able to identify a unique 2-isoxazoline-3-carboxylate substrate that demonstrated desired reactivity and formed our target azetidine product in 69% yield.
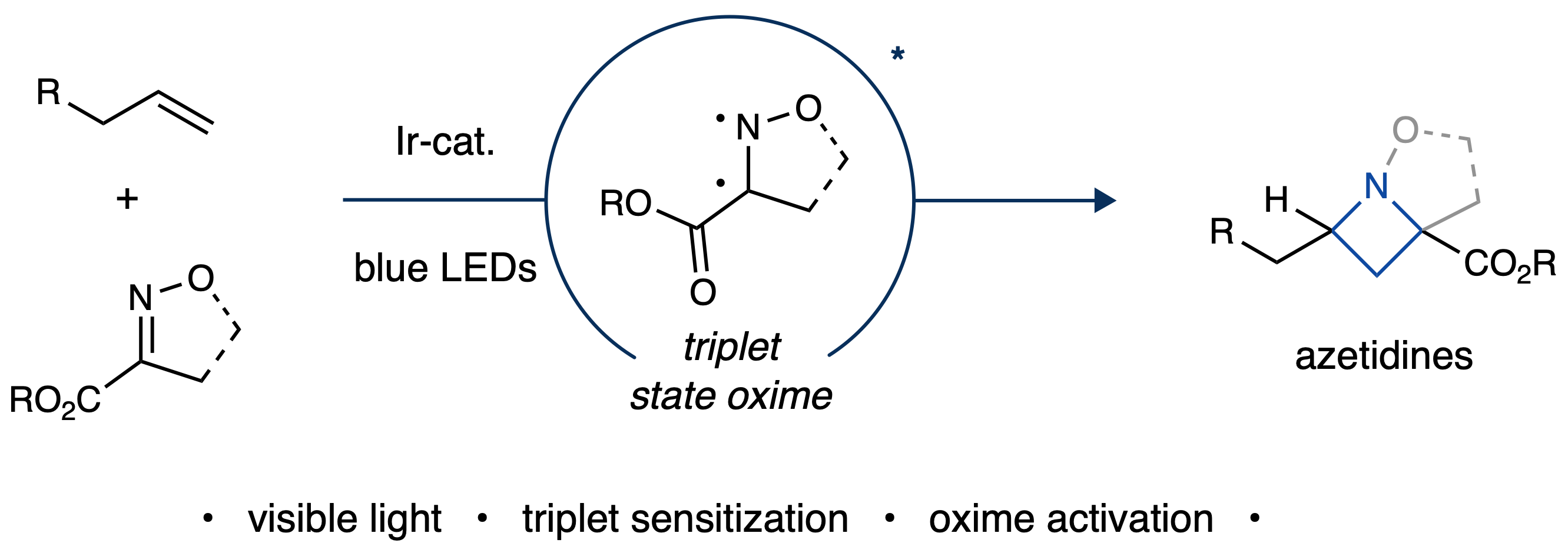
After reaction optimization, we were able to obtain substantially increased yields (up to 99%) by using a different iridium photocatalyst, and were subsequently able evaluate the scope of this reaction. We were surprised that most alkenes we tested (see Supplementary Information) were well tolerated, which allowed us to form a wide range of substituted and functionalized azetidine products. This regioselectivity was generally very high, even for tri-substituted alkenes, albeit modest diastereoselectivity was observed.
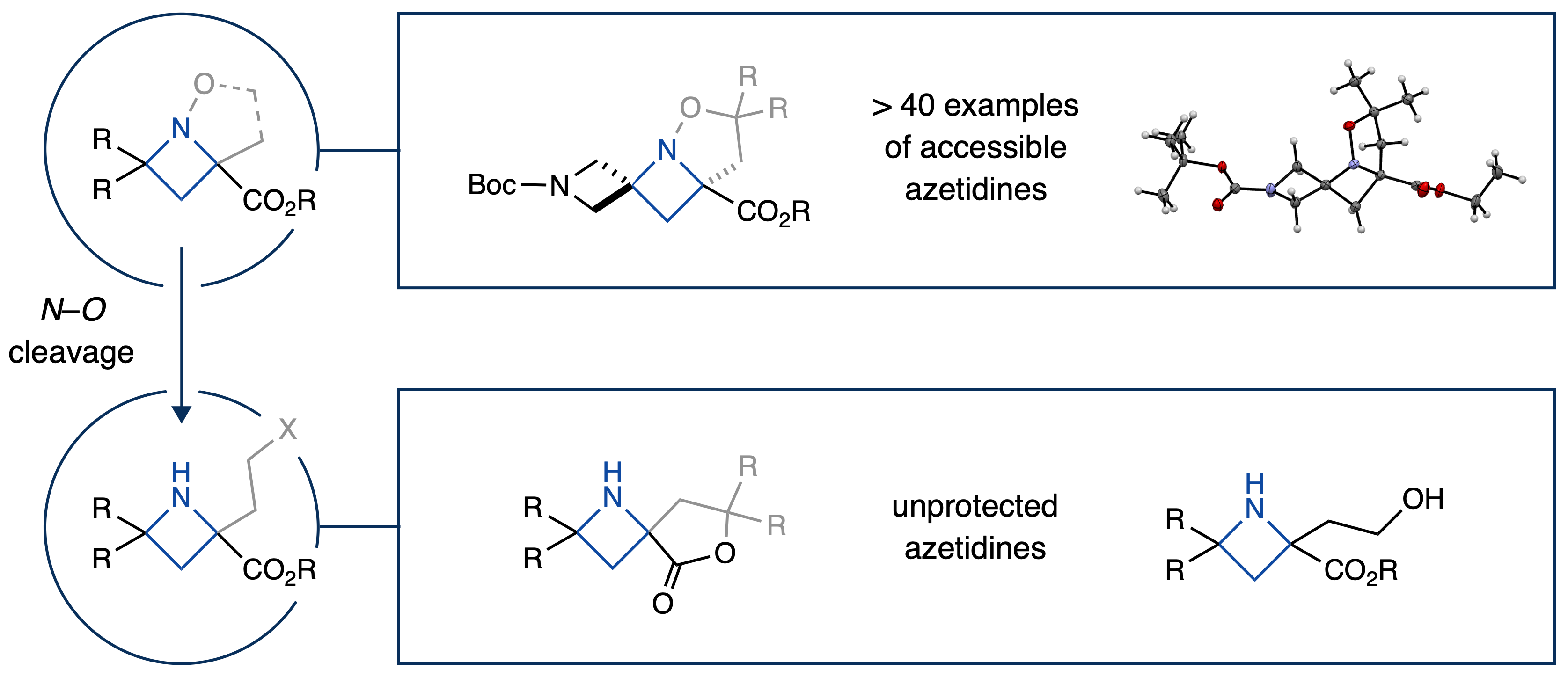
However, we knew the broad synthetic utility of this new method relied on access to free azetidines from our initial bicyclic products, leaving us with the final task of determining suitable conditions for N–O cleavage. To achieve this goal, we extensively screened reduction conditions for N–O bond cleavage and found just the right combination of catalyst, acid, and solvent for our hydrogenolysis reaction to efficiently yield a variety of free azetidines. We were excited with this result, as it represents a facile method to access free azetidines in a simple, user friendly, and atom economical fashion. We anticipate that this work will allow for further exploration of azetidines in both synthesis and drug discovery applications!
Interested in learning more about our intermolecular aza Paternò-Büchi method?
See the full report in Nature Chemistry here:
DOI: 10.1038/s41557-020-0541-1
Follow the Topic
-
Nature Chemistry

A monthly journal dedicated to publishing high-quality papers that describe the most significant and cutting-edge research in all areas of chemistry, reflecting the traditional core subjects of analytical, inorganic, organic and physical chemistry.




Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in