Tandem amine scrubbing and CO2 electrolysis via direct piperazine carbamate reduction
Published in Chemistry, Earth & Environment, and Materials
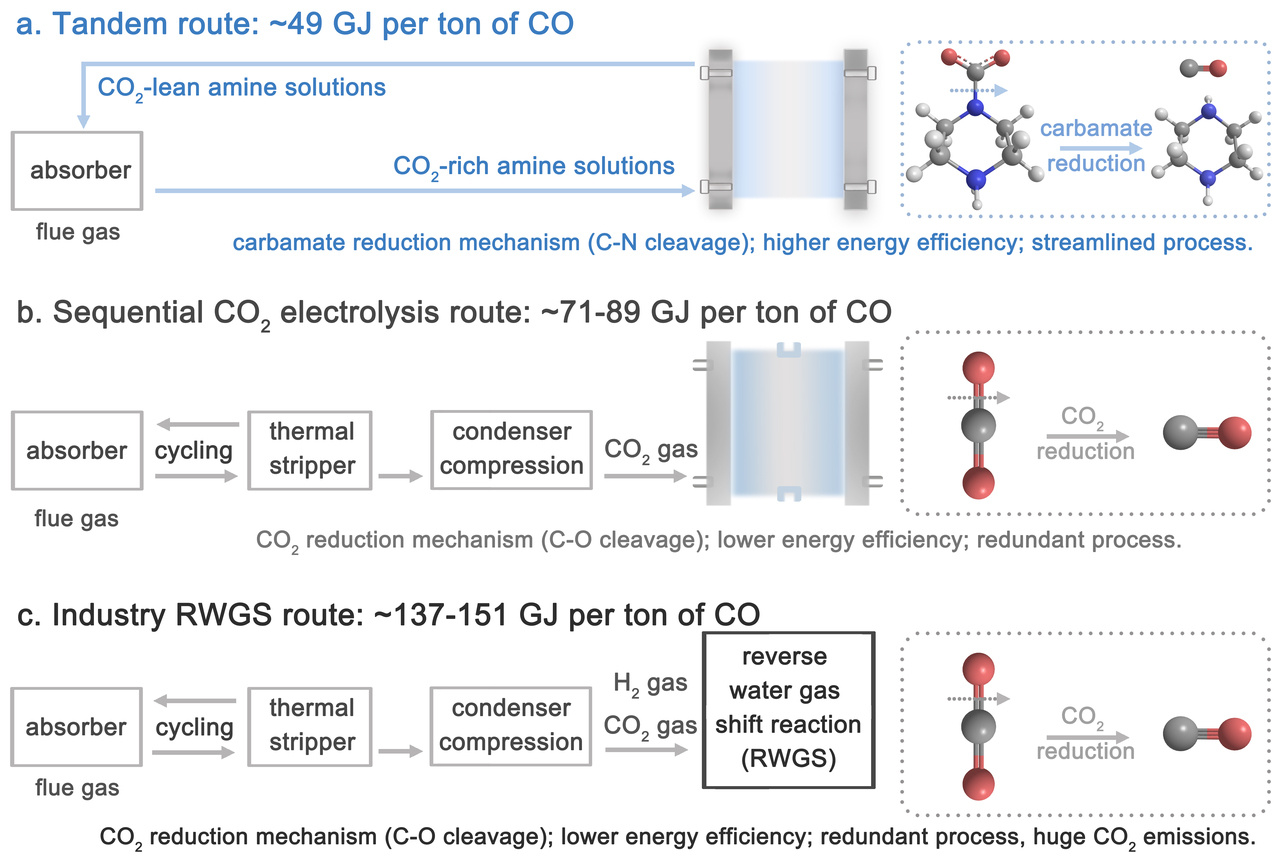
(1) Capturing CO2 and upgrading it in one electrified step.
Post-combustion capture with amines is mature, but traditionally you must reboil the solvent to release CO2 before any conversion — an energy-hungry detour. In our Nature Energy (https://www.nature.com/articles/s41560-025-01869-8) paper, we show that you can take the captured carbamate directly from an amine (piperazine), feed it to an electrolyser, and convert it into CO while regenerating the amine. A nickel single-atom catalyst in a zero-gap AEM cell acts like a molecular “switchboard,” cleaving the right bond so the solvent is refreshed, and carbon becomes a useful building block. The result is a simpler, more energy-lean loop that points to practical, integrated CO2 capture and utilization.
(2) The five months that nearly washed us away.
Our first-round peer review arrived with an entirely lab challenge: the unexpected lab ceiling flooding issues shut down the lab for three months. Just as we were preparing the revision, the lab ceiling decided to test our resilience—with an unexpected flood that shut us down for three months. During this “forced sabbatical,” we survived on paperwork and borrowed instruments from friendly neighbours. Once the waters receded, we returned to the bench, wrapped up the experiments, and finally delivered the full revision package. Armed with this, we delivered the reviewers what they asked for: stable, selective operation with the integrated loop, multiple cycles of amine regeneration and CO production at lower CO2 loading conditions, and data that reflected not a lucky afternoon but a robust process window. Looking back, the “flooding months” were less a detour than the road itself — they taught us what an integrated capture-electrolysis system needs to behave in the real world.
(3) What comes next: from a clever unit to a whole system.
Our paper shows that the chemistry works and that a carefully managed device can run steadily. The future is to make the system sing. First, we’ll connect a working absorber/stripper line (or lean amine loop) directly to the electrolyser, managing thermal and material recirculation so that waste heat and by-product streams are put to work. The goal is a single, balanced loop where the solvent is continuously refreshed electrochemically. Second, real flue-gas readiness. Industrial CO2 arrives with SOx/NOx, O2, and humidity. We’ll quantify tolerance, add pre-polish steps only where needed, and stress-test the catalyst and membrane over hundreds of hours in impurity-realistic conditions.
If the paper is a blueprint for the key reaction step, our next chapter is about wiring the house — plumbing, sensors, feedback, and the economics that determine whether this becomes a standard option for decarbonizing point sources.
Follow the Topic
-
Nature Energy

Publishing monthly, this journal is dedicated to exploring all aspects of this on-going discussion, from the generation and storage of energy, to its distribution and management, the needs and demands of the different actors, and the impacts that energy technologies and policies have on societies.
Your space to connect: The Fuel cell technologies Hub
A new Communities’ space to connect, collaborate, and explore research on Electrochemistry, Chemical Engineering, and Fuel Cells!
Continue reading announcementRelated Collections
With Collections, you can get published faster and increase your visibility.
Microgrids and Distributed Energy Systems
Publishing Model: Hybrid
Deadline: Mar 31, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in