Targeting serine/glycine metabolism improves radiotherapy response in Non-Small Cell Lung Cancer
Published in Cancer

The sugar rush of lung cancer cells
Cancer cells have adapted in multiple ways to grow and spread within the human body. In order to enable this growth and the progression of the cancer, cancer cells need sufficient amounts of nutrients and, therefore, they have high requirements for metabolites. These metabolites can be obtained from food intake, yet, cancer cells, owing to their elevated requirements and inaccessibility to these metabolites, find it more advantageous to supply themselves with the required metabolites via intracellular production or so-called synthesis. This metabolic adaptation process is known as metabolic rewiring, which is a common hallmark of cancer cells (1).
Lung cancer is the most lethal cancer and about 85% of lung cancers are classified as non-small cell lung cancer (NSCLC) (2). Therapeutic resistance to the current standard-of-care chemo-radiotherapy remains a major challenge in the treatment of this cancer. We know that NSCLCs are metabolically rewired and very reliant on metabolites for their high requirements to grow, which also relates to tumor aggressiveness and, therefore, therapy resistance and poor patient outcomes. Many genetic alterations in NSCLC, such as NKX2-1 amplifications and common LKB1 or KEAP1 mutations in NSCLC tumors, lead to serine and glycine pathway metabolic rewiring (3-5). While normal lung cells take up serine and glycine from their environment, these lung cancer cells can synthesize their own serine and glycine from the sugar glucose, which is more commonly available in the microenvironment of cancer cells (Figure 1).
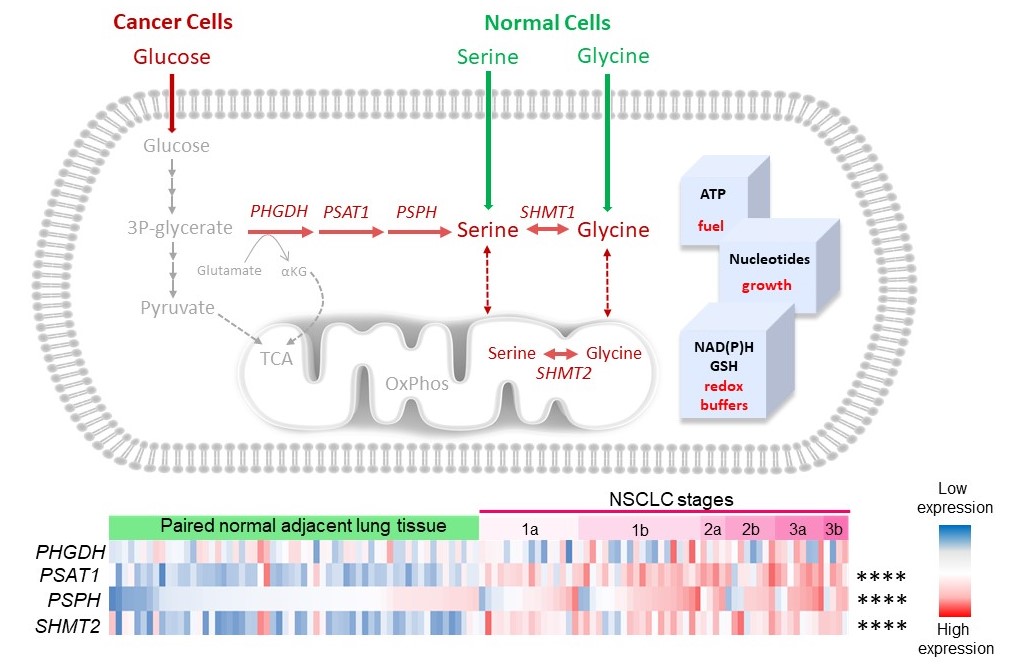
Figure 1. Heatmap used to visualize the expression of serine/glycine pathway synthesis enzymes, i.e. PHGDH, PSAT1, PSPH and SHMT2 by RNA-sequencing in NSCLC patient tumors compared to the paired normal adjacent lung tissue. The log2 transformed data was collected from R2 AMC genomics platform using the publicly available (GSE19804) dataset. Fisher’s exact test was used to calculate the frequency of samples with high expression in NSCLC or normal paired tissue samples, calculated as median log2 expression. ****p-value <0.0001.
Figure 1 illustrates that normal cells hardly express any of the serine/glycine pathway enzymes (blue), while the lung cancer cells from the same patient show a clear enhanced expression of the serine/glycine pathway enzymes (red). This metabolic adaptation provides the NSCLC tumors with sufficient amounts of serine and glycine, which the cancer cells use to generate ATP and nucleotides, the essential building blocks of cancer cells for their replication and growth. Furthermore, this production system also provides redox buffers, allowing the cancer cells to become more therapy resistant, as most therapeutic agents cause cellular oxidative stress, which is neutralized by these redox buffers, such as glutathione (GSH). Collectively, this serine/glycine metabolic rewiring, which occurs in 37% of the NSCLC tumors, is linked to therapeutic failure, leading to poor prognosis in NSCLC patients.
In this study, we challenged to landscape metabolic changes in NSCLC in response to radiotherapy and test the efficacy of simultaneously targeting the serine/glycine metabolic adaptations for the treatment of NSCLC. To target serine/glycine metabolism, we make use of our previously identified and repurposed serine/glycine conversion SHMT inhibitor, sertraline (6). Sertraline, also known as Serlain, Lustral, Besitran or Zoloft, is originally described as anti-depressant.
Key findings
In NSCLC patients and cell models, we observed that radiotherapy treatment reduces glycolysis pathway metabolites as well as serine and glycine pathway metabolites. Intrigued by this finding, we wondered about the reason behind the observed decrease in these metabolite levels in response to radiotherapy. With 13C-glucose tracing experiments, we could detect that lung cancer cells consume more serine/glycine pathway metabolites and/or start producing more serine/glycine pathway-derived metabolites to recover from radiotherapy targeting. This observation indicated that the serine/glycine pathway provides the metabolites that help the cancer cells recover and become more resistant to radiotherapy. We looked further into this by analyzing the expression of serine/glycine conversion enzyme SHMT2. In NSCLC cell models, we could pinpoint that the lung cancer cells induce expression of SHMT2 serine-glycine converting enzyme in response to radiotherapy (Figure 2A). Using our isogenic NKX2-1 driven lung cancer model, we further confirmed that serine/glycine pathway dependent NKX2-1 lung cancer cells are more resistant to radiotherapy treatment compared to the same cells that lack NKX2-1 expression (Figure 2B).
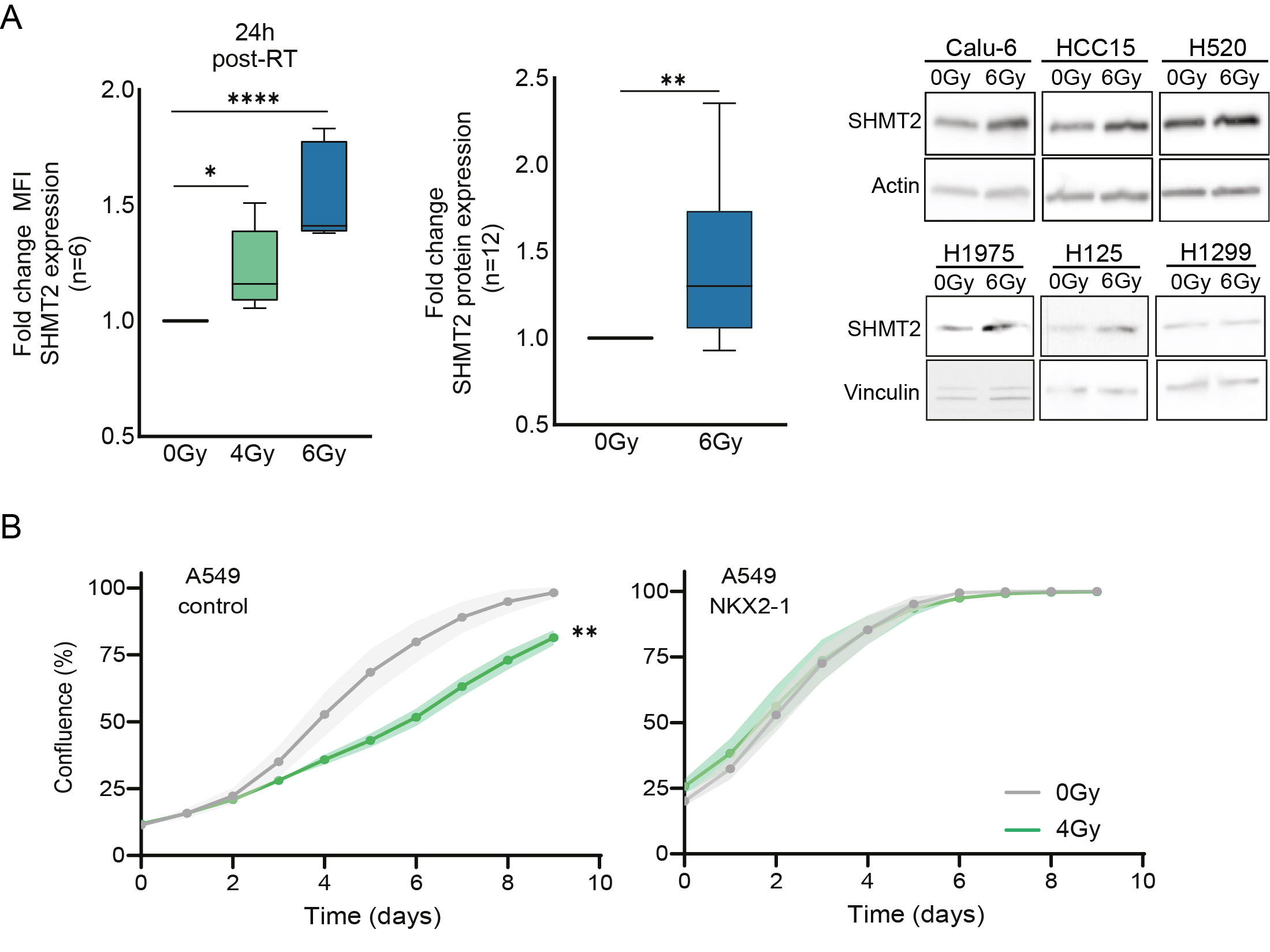
Figure 2. A) Fold change of mean fluorescence intensity (MFI) of SHMT2 protein expression using flow cytometry in NSCLC cell lines (n=6) in response to irradiation, 4Gy and 6Gy for 6 and 24 hours. An unpaired 2-tailed t test was used to compare irradiated cells vs control cells. Fold change of SHMT2 protein expression measured by immunoblot in n=6 NSCLC cell lines 24h post-irradiation and n=2 biological replicate for each cell line. An unpaired 2-tailed t test has been used to compare irradiated cells and control cells. B) Cell growth curves showing the confluence (%) of A549 control cells and A549 cells with overexpression of NKX2-1 upon exposure to 4 Gy irradiation. Fold change of area under the curve (AUC) of the A549 control NKX2-1 overexpression cell growth curves. Each dot represents an independent experiment. Unpaired 2-tailed t-test has been performed. Statistical analysis *p-value < 0.05, **p-value < 0.01, ****p-value < 0.0001.
With sertraline identified as a serine/glycine conversion SHMT inhibitor, we explored whether sertraline treatment could re-sensitize NSCLC cells to radiotherapy. Using different 2D and 3D cancer stem cell-like assays, we demonstrated that sertraline combined with radiotherapy synergistically targets the lung cancer cell clonogenic, spheroid, and proliferative capacity, even weeks after the treatment was removed (Figure 3). This combination therapy showed efficacy above expectations; therefore, we were interested in testing this therapeutic approach in a lung cancer animal model.
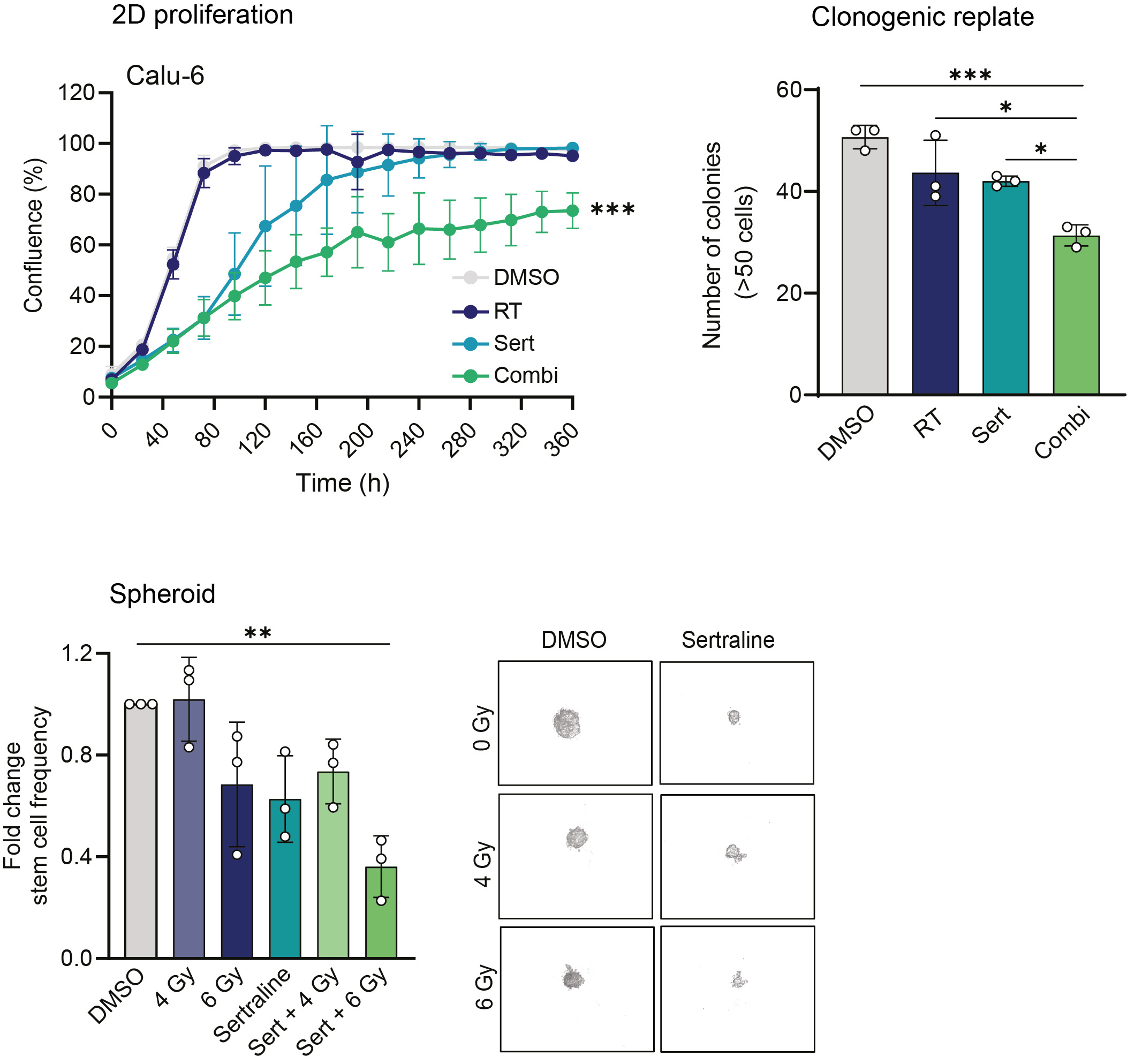
Figure 3. Proliferation curves of NSCLC Calu-6 cell line was treated with 5 µM sertraline and 6 Gy irradiation. Data are presented as mean ± SD of 6 replicate wells and are representative of n≥3 independent experiments. Graph showing the number of colonies (>50 cells) after replating 500 cells from end-stage clonogenic surviving colonies of treated Calu-6 cell line. One-way ANOVA with Tukey’s multiple comparison test was used. Data are represented as mean ± standard deviation. Individual dots represent different technical replicates. Graph representing the spheroid assay and showing the fold change in stem cell frequency of the different originating groups compared to the control cells. One-way ANOVA with Dunnett's multiple comparisons test was used. Data are represented as mean ± standard deviation. Individual dots represent n=3 independent calculations from limiting dilution spheroid experiments. Representative pictures at 20x magnification of Calu-6 spheres at day 30 after an initial seeding density of 25 cells/well and condition. Statistical analysis *p-value < 0.05, **p-value < 0.01, ***p-value < 0.001.
In NSCLC patients, we made an interesting observation that, at diagnosis, blood plasma serine/glycine pathway metabolite levels show an inverse correlation with baseline lymphocyte counts. These high serine/glycine-associated low baseline lymphocyte counts also indicate that these NSCLC patients will respond poorly to immunotherapy (7). To test the link between serine/glycine metabolism and immune cell responses in NSCLC, we exploited our isogenic NKX2-1 lung cancer model. Here, we demonstrated that serine/glycine pathway dependent NKX2-1 lung cancer cells suppress immune cells by 50% more than the same lung cancer cells that lack NKX2-1. These data together imply the need for a mouse lung cancer model in order to test the efficacy of sertraline combined with radiotherapy on tumor growth as well as on the immune system. Therefore, we made use of the Lewis lung cancer model, cultured for 2 days in serine/glycine deprived media to metabolically rewire these lung cancer cells towards serine/glycine metabolism. Furthermore, this Lewis lung cancer model is known to be very aggressive and non-responsive to immune checkpoint inhibitor treatments, even when combined with radiotherapy (8). Dosages used in this animal study reflect the maximum currently used clinical dosages for anti-depressant prescriptions, yet we administered the dosage every three days instead of providing a continuous daily dosage.
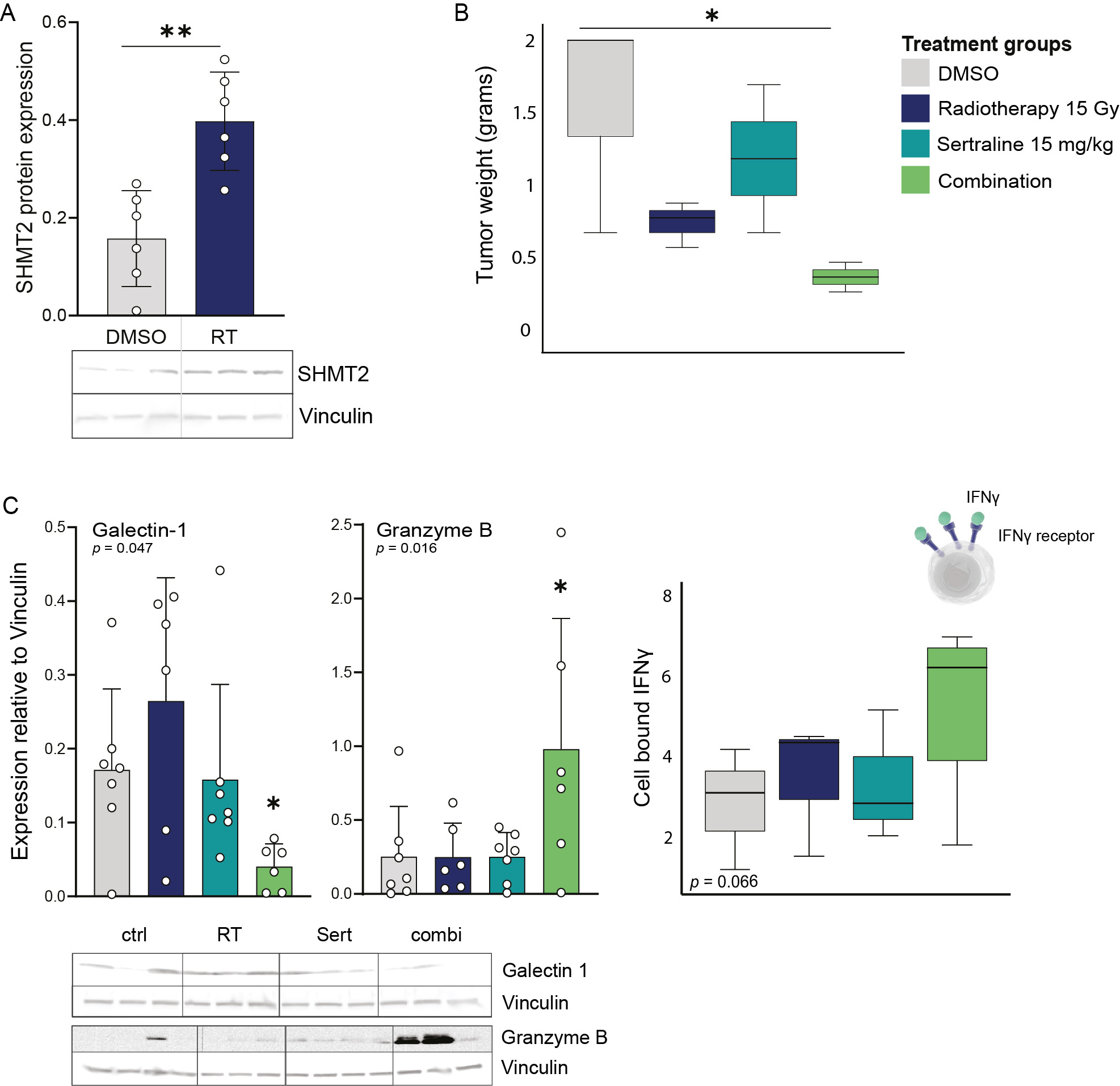
Figure 4. A) SHMT2 protein expression on day 17 measured by immunoblot in n=3 LLC tumors and n=2 technical replicates. An unpaired two-tailed t test has been used to compare DMSO treated mice and irradiated tumors. B) Box plot showing tumor growth as tumor weight in the different treatment groups: DMSO, RT 15 Gy, sertraline 15mg/kg, and combination. Kruskal-Wallis test with a Dunn's multiple comparisons test was performed. C) Galectin-1 and granzyme B protein expression measured by immunoblot in the tumors of n≥3 mice for each treatment group and n=2 technical replicates. A Jonckheere-Terpstra test was performed. Box plot showing the % of cell bound IFN-γ measured by flow cytometry in dissociated tumors collected from mice treated with DMSO, RT, sertraline, and combination treatment. Statistical analysis *p-value < 0.05, **p-value < 0.01.
Validating our previous findings, the animal group that received radiotherapy showed a clear induced expression of serine/glycine converting enzyme SHMT2 in the tumors compared to control mice (Figure 4A). Neoadjuvant sertraline treatment to radiotherapy could effectively reduce and control tumor growth in this Lewis lung cancer model, while neither radiotherapy nor sertraline treatment alone showed effects on tumor weights (Figure 4B). The combination therapy effectively blocked systemic serine/glycine pathway metabolites, indicated by reduced glycine blood serum levels and a simultaneous accumulation of homocysteine, which cannot be further processed into glutathione when lacking glycine. In mice treated with the combination therapy, we observed a remodeling of the immune landscape, characterized by increasing amounts of CD11-expressing NK cells with enhanced expression of IFNγ and granzyme B (Figure 4C). The most important finding in our combination therapy group was the decreased levels of immune suppressive checkpoint Galectin-1 (Figure 4C). This may reflect the lack of response in Lewis lung cancer model to PD-1/PD-L1 immune checkpoint inhibitors combined with radiotherapy, as well as it may also support why still ~50% of NSCLC patients do not respond to current clinically standard-of-care usage of PD-1/PD-L1 immune checkpoint inhibitors.
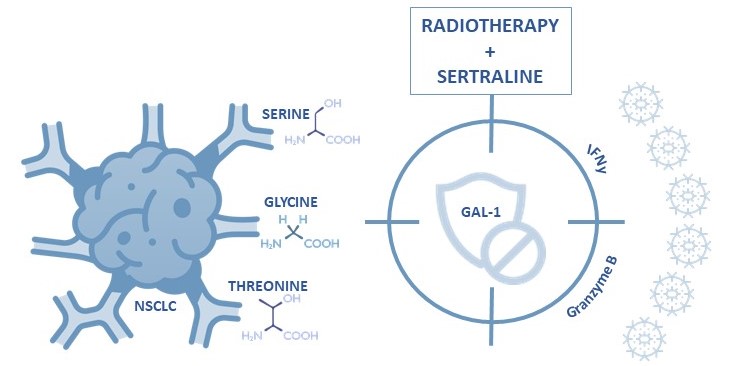
Figure 5. Serine/glycine pathway metabolite dependent NSCLC can be effectively inhibited and targeted by radiotherapy plus neoadjuvant sertraline treatment to overcome galectin-1 mediated immune evasion.
Take home message
Neoadjuvant sertraline treatment with radiotherapy shows promising results in overcoming galectin-1-associated immune suppressive phenotypes of NSCLC (Figure 5). This knowledge is very important to improve future immunotherapy strategies for cancer patients. With this study, we challenge to revisit current therapeutic approaches and improve cancer patient’s outcomes by implicating neoadjuvant sertraline treatment.
Regarding our research, we will proceed to investigate the role of serine/glycine pathway rewiring across cancers to enable a better stratification approach for cancer patients who may benefit from neoadjuvant sertraline treatment. In the supplementary data of our study, we show that the radiotherapy induced SHMT2 expression and dependency is not restricted to NSCLC, but is also observed in, for example, radiotherapy-treated multiple myeloma cell models. This highlights the broad novelty for implementation of neoadjuvant sertraline treatment in the clinic for cancer patients.
- Hanahan D. Hallmarks of cancer: new dimensions. Cancer discovery. 2022;12(1):31-46.
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446-54.
- Heylen E, Verstraete P, Van Aerschot L, Geeraerts SL, Venken T, Timcheva K, et al. Transcription factor NKX2–1 drives serine and glycine synthesis addiction in cancer. British Journal of Cancer. 2023:1-17.
- Kottakis F, Nicolay BN, Roumane A, Karnik R, Gu H, Nagle JM, et al. LKB1 loss links serine metabolism to DNA methylation and tumorigenesis. Nature. 2016;539(7629):390-5.
- DeNicola GM, Chen P-H, Mullarky E, Sudderth JA, Hu Z, Wu D, et al. NRF2 regulates serine biosynthesis in non–small cell lung cancer. Nature genetics. 2015;47(12):1475-81.
- Geeraerts SL, Kampen KR, Rinaldi G, Gupta P, Planque M, Louros N, et al. Repurposing the antidepressant sertraline as SHMT inhibitor to suppress serine/glycine synthesis–addicted breast tumor growth. Molecular cancer therapeutics. 2021;20(1):50-63.
- Karantanos T, Karanika S, Seth B, Gignac G. The absolute lymphocyte count can predict the overall survival of patients with non-small cell lung cancer on nivolumab: a clinical study. Clinical and Translational Oncology. 2019;21:206-12.
- Pimentel VO, Marcus D, van der Wiel AM, Lieuwes NG, Biemans R, Lieverse RI, et al. Releasing the brakes of tumor immunity with anti-PD-L1 and pushing its accelerator with L19–IL2 cures poorly immunogenic tumors when combined with radiotherapy. Journal for immunotherapy of cancer. 2021;9(3).
Follow the Topic
-
British Journal of Cancer

This journal is devoted to publishing cutting edge discovery, translational and clinical cancer research across the broad spectrum of oncology.



Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in