

Note:CERT transfer ceramide from the endoplasmic reticulum (ER) to the Golgi apparatus, preventing its apoptosis-inducing accumulation and thereby promoting cell survival. This biological process parallels the legendary story of Yu Gong, who persistently moved mountains obstructing his village to ensure convenient transportation for future generations.
Background
Sphingomyelin (SM), ceramide (Cer), and glycosphingolipids constitute essential structural components of cell membranes, forming sphingolipid-enriched microdomains that regulate cancer cell signaling pathways (1, 2). Notably, Cer has been widely recognized as a tumor suppressor across various cancer types (3, 4), primarily through its central role in mediating apoptosis (5). In contrast, SM has been demonstrated to be crucial for supporting cancer cell proliferation, migration, and immune evasion (6, 7). The intracellular balance between Cer and SM is precisely regulated by ceramide transfer protein (CERT), the exclusive lipid transporter responsible for Cer trafficking from the endoplasmic reticulum to the Golgi apparatus.
Recent investigations have revealed significantly reduced Cer levels in primary cells from FLT3-positive acute myeloid leukemia (AML) patients, while FLT3 inhibitors have shown therapeutic potential by elevating Cer concentrations and inducing apoptosis in AML cells (8). Although the development of FLT3 inhibitors has revolutionized targeted therapy for AML, clinical challenges persist, particularly in FLT3-mutated patients who frequently experience high relapse rates, poor prognosis, and developing drug resistance. These limitations underscore the urgent need to identify novel therapeutic targets that can enhance FLT3 inhibitor sensitivity and develop innovative combination strategies incorporating FLT3 inhibitors. In this context, targeting CERT emerges as a promising anti-cancer approach with significant potential for both scientific advancement and clinical application in AML treatment.
Results
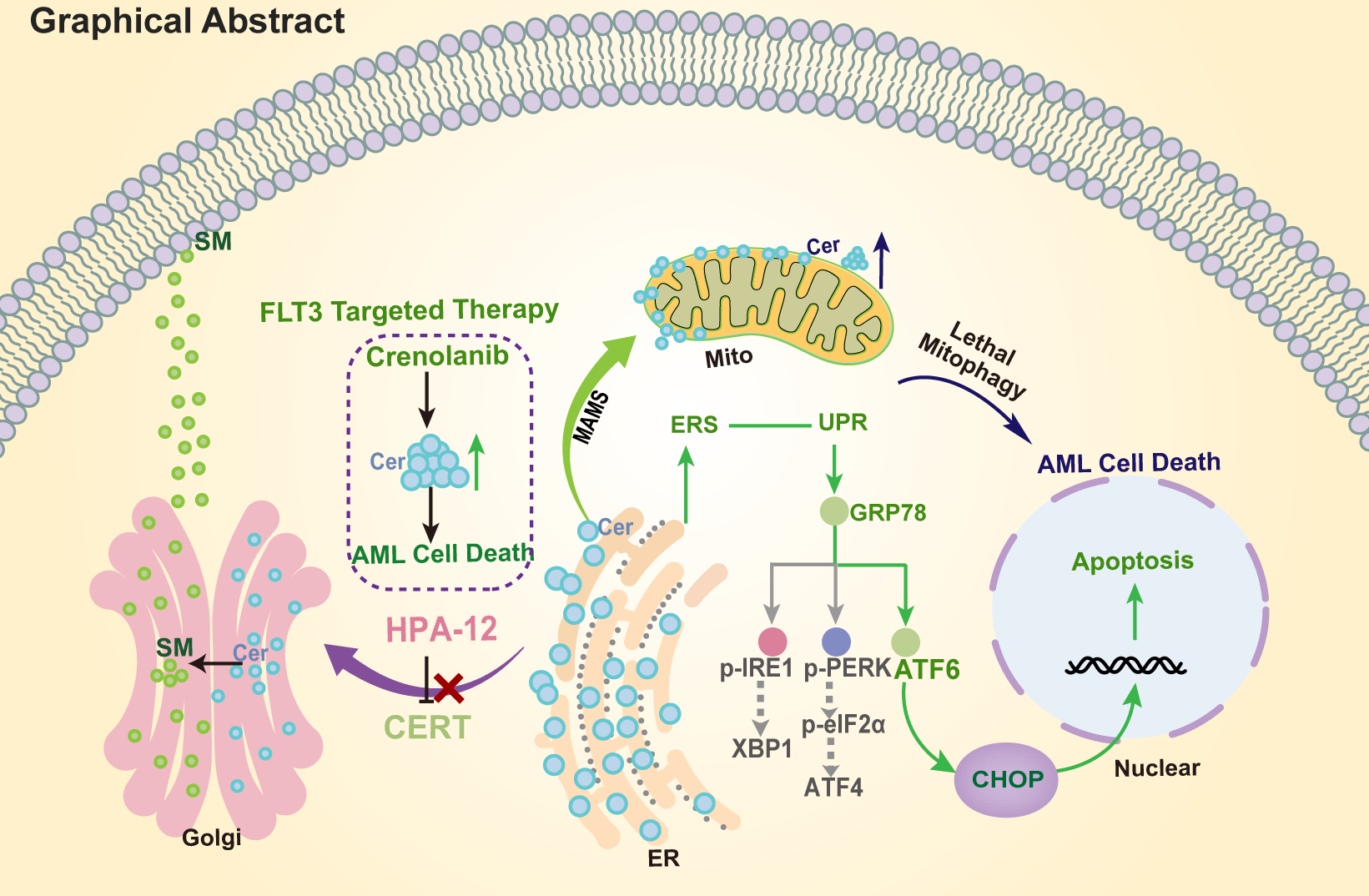
We first determined the expression level of CERT in various AML cell lines, and found that the protein level of cert was higher in AML cell lines with FLT3-ITD mutation. Targeting CERT at the genetic and pharmacological levels can inhibit cell growth and induce cell apoptosis. Moreover, inhibition of CERT can increase the sensitivity of FLT3 mutant AML cell line to FLT3 inhibitors, synergistically inhibit the proliferation of AML cells and induce apoptosis. The combination of these two drugs induced the accumulation of Cer and blocked the intracellular transport of Cer, inducing the occurrence of ER stress, which subsequently activated unfolded protein response (UPR) to inhibit the growth of AML cells and induce apoptosis. By knocking down the GRP78, ATF6 and CHOP genes in AML cells, we verified the synergy of CERT inhibitors and FLT3 inhibitors was through the activation of GRP78/ATF6/CHOP axis.
To further study the role of CERT in FLT3 mutation AML, we established a CDX model using MV4-11-luc+cells. The results showed that the combination of CERT inhibitor and FLT3 inhibitor in the animal model inhibited the development of AML disease in mice and prolonged the survival of diseased mice.
In conclusion, this study reveals the molecular mechanism of the important role of the GRP78/ATF6/CHOP axis in mediating the death of AML cells induced by the combination of CERT inhibitor and FLT3 inhibitor. This work shows that the combination of CERT inhibitors with FLT3 inhibitors may be an effective way to overcome the resistance of FLT3 inhibitors in AML, which provides a potent target and a combination scheme to reduce recurrence and drug resistance.
Take home message
We have provided evidence for strategies targeting the clearance of Cer to treat AML, especially for patients with FLT3 mutations. An inhibitor of the Cer trafficking protein CERT in collaboration with FLT3 inhibitors may represent a promising combinatory regimen to reduce the possibility of relapse and resistance.
Reference
1 Schömel, N., Geisslinger, G. & Wegner, M. S. Influence of glycosphingolipids on cancer cell energy metabolism. Progress in lipid research 79, 101050, doi:10.1016/j.plipres.2020.101050 (2020).
2 Canals, D. & Clarke, C. J. Compartmentalization of Sphingolipid metabolism: Implications for signaling and therapy. Pharmacology & therapeutics 232, 108005, doi:10.1016/j.pharmthera.2021.108005 (2022).
3 Morad, S. A. & Cabot, M. C. Ceramide-orchestrated signalling in cancer cells. Nature reviews. Cancer 13, 51-65, doi:10.1038/nrc3398 (2013).
4 Bai, A. P. & Guo, Y. Ceramide is a Potential Activator of Immune Responses Against Tumors. Gastroenterology 155, 579-580, doi:10.1053/j.gastro.2018.04.037 (2018).
5 Hannun, Y. A. & Obeid, L. M. Sphingolipids and their metabolism in physiology and disease. Nature reviews. Molecular cell biology 19, 175-191, doi:10.1038/nrm.2017.107 (2018).
6 Tallima, H., Azzazy, H. M. E. & El Ridi, R. Cell surface sphingomyelin: key role in cancer initiation, progression, and immune evasion. Lipids in health and disease 20, 150, doi:10.1186/s12944-021-01581-y (2021).
7 Haddadi, N., Lin, Y., Simpson, A. M., Nassif, N. T. & McGowan, E. M. "Dicing and Splicing" Sphingosine Kinase and Relevance to Cancer. International journal of molecular sciences 18, doi:10.3390/ijms18091891 (2017).
8 Dany, M. et al. Targeting FLT3-ITD signaling mediates ceramide-dependent mitophagy and attenuates drug resistance in AML. Blood 128, 1944-1958, doi:10.1182/blood-2016-04-708750 (2016).
Follow the Topic
-
Nature Communications

An open access, multidisciplinary journal dedicated to publishing high-quality research in all areas of the biological, health, physical, chemical and Earth sciences.
Related Collections
With Collections, you can get published faster and increase your visibility.
Women's Health
Publishing Model: Hybrid
Deadline: Ongoing
Advances in neurodegenerative diseases
Publishing Model: Hybrid
Deadline: Mar 24, 2026





Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in